Rapamycin structural analogue and preparation method thereof
A technology of methyl rapamycin and crude extract, applied in the field of combined biosynthesis, can solve problems such as stereoisomerism, and achieve the effects of good antifungal effect, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
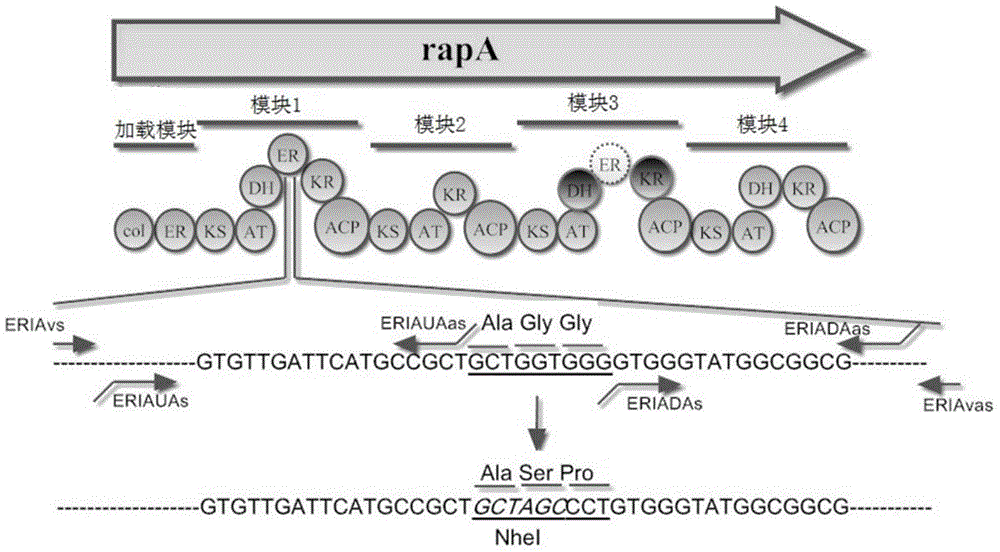
[0041] Structural analysis of functional domains of polyketide synthase rapA from Actinomycetes molluscs.
[0042]Using http: / / nrps.igs.umaryland.edu / nrps / _ to predict the functional domain of polyketide synthase rapA, the results are shown in Table 1:
[0043] Table 1
[0044]
[0045] The rapA gene contains a loading module and four chain extension modules, and the layout of each module is shown in figure 1 shown. Act_rapA refers to Actinomycetes rapA gene, CL is carboxylic acid ligase, AT is acyltransferase domain, KS is ketosynthase domain, DH is dehydrogenase domain, ER is enoyl reductase domain , KR is the ketoreductase functional domain, and ACP is the acyl carrier protein functional domain.
Embodiment 2
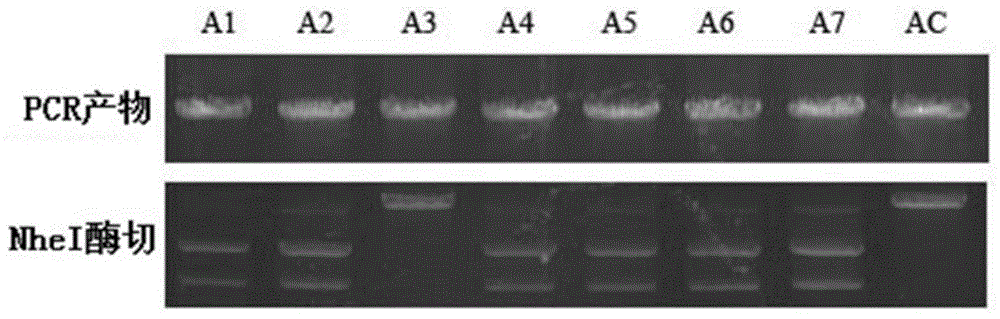
[0047] Obtaining a homologous double-crossover strain with a point mutation in the ER functional domain of Actinomycetes mobilis rapA.
[0048] The schematic diagram of the point mutation of the ER functional domain of Actinomycetes rapA is shown in figure 1 . Two pairs of primers ERIAUs-ERIAUas and ERIADAs-ERIADAas were used to amplify the two fragments upstream and downstream of the mutation site of the ER functional domain of Actinomyces mobilis rapA gene, respectively, and then the restriction endonuclease contained in the introduced mutation site NheI joins the two fragments.
[0049] A) Extraction of Actinomycetes mobilis N902-109 genome:
[0050] Take a small amount of mycelium of Actinomycetes mobilis N902-109 and inoculate it into a test tube containing 5 mL of YEME medium, and culture it with shaking at 30°C for about 72 hours. The mycelium was collected by centrifugation at 3500 rpm, washed twice with TES (10 mM Tris-HCl pH 7.5, EDTA 1 mM, NaCl 50 mM). Add 1mL T...
Embodiment 3
[0085] Preparation and Identification of Structural Analogues of Rapamycin by Fermentation
[0086] A) Fermentation of Actinomycetes N902-109ERIA
[0087] Put the actinomycetes N902-109ERIA droplet preserved in the glycerin tube into the slant of the actinomycetes fermentation slant medium, spread it evenly with an inoculation rod, and culture at a constant temperature of 28°C for 7-9 days. Use the inoculation shovel to scrape the slant mycelia and insert it into the 30ml first-level seed culture medium, and at 28° C., 220 revolutions for constant temperature cultivation for 4-5 days, then receive the second-level seed culture medium by 10% of the inoculum size, and the percentage is accounted for. The volume percentage of secondary seed culture medium volume, 28 ℃ 220 rpm constant temperature culture 3-4 days, then receive in the fermentation bottle that secondary seed culture medium is housed according to inoculum size 7.5%-10%, described percentage is Accounting for the vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com