Fusion protein, fusion protein preparation method and porcine oral vaccine or medicine
A fusion protein and protein technology, applied in biochemical equipment and methods, fusion peptides, drug combinations, etc., can solve problems such as limited vaccine effects and achieve the effect of protecting diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment illustrates the preparation method of recombinant Omp16-PEDVS / ATCC393 Lactobacillus lactis (oral vaccine used in Example 3), comprising the following steps:
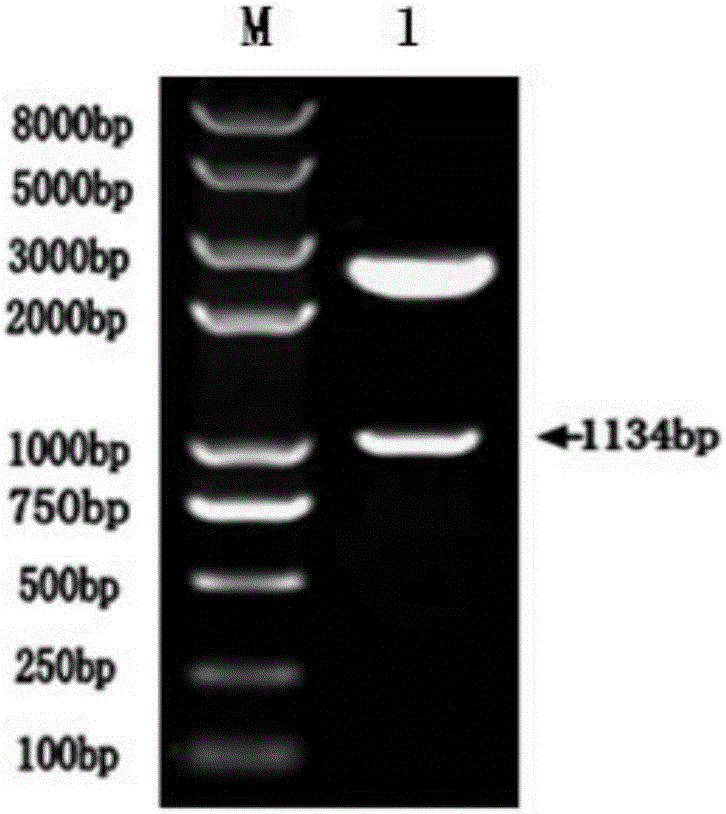
[0047] 1. Synthesize the OMP16-PEDVS gene, add a Sal I restriction endonuclease site at the 5' end, and add an EcoRV restriction endonuclease site at the 3' end. The synthetic gene is cloned on the pUC57 vector and named as pUC57-Omp16-PEDVS (the sequence of its fusion protein is shown in sequence SEQ.No.2), the Sal I and Eco RV double enzyme digestion identification results of pUC57-Omp16-PEDVS plasmid are as follows figure 2 shown.
[0048] 2. Transformation of ATCC393 Lactobacillus lactis with pVE5523-Omp16-PEDVS plasmid
[0049] The correctly sequenced pUC57-Omp16-PEDVS plasmid and the pVE5523 expression vector plasmid were digested with Sal I and Eco RV, the target fragment and the pVE5523 vector fragment were recovered from the gel, ligated with T4Ligase (T4DNA ligase), and the ligated prod...
Embodiment 2
[0058] This embodiment illustrates the preparation method of recombinant Omp16-PEDVS and PEDVS prokaryotic expression protein (two kinds of injection vaccines used in embodiment 3), comprising the following steps:
[0059] 1. Construction of pET28a-OMP16-PEDVS expression vector
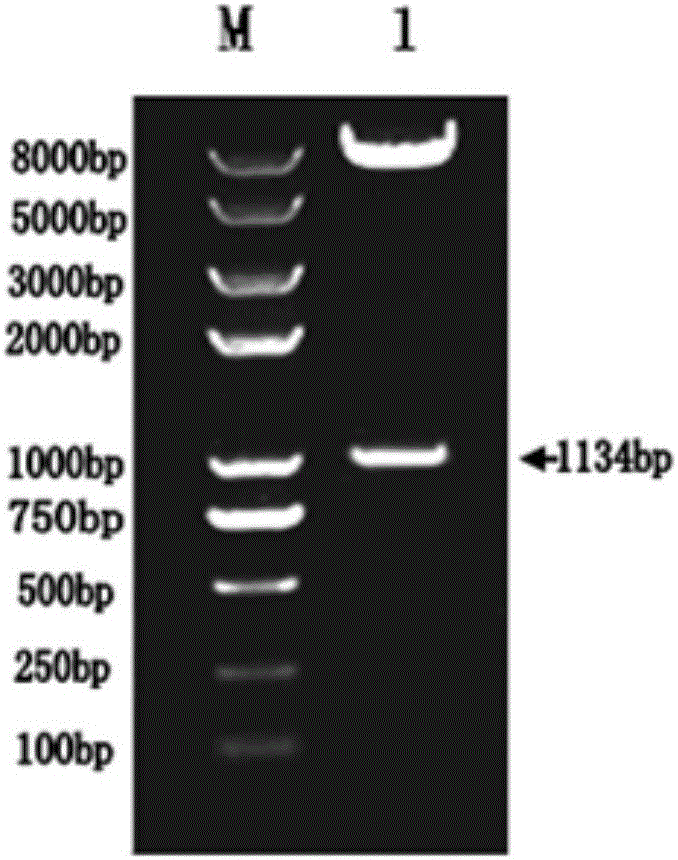
[0060] Using the pVE5523-OMP16-PEDVS gene as a template, design the upstream primer F1 (see sequence SEQ.No.3) and downstream primer R1 (see sequence SEQ.No.4) for amplifying OMP16-PEDVS, and add Nco I restriction endonuclease site, and 3' plus Xho I restriction endonuclease site, the gene was subcloned into pET28a(+) prokaryotic expression vector, named pET28a-OMP16-PEDVS. The result is as Figure 4 1, and transform the correct plasmid into BL21(DE3) E. coli.
[0061] 2. Construction of pET28a-PEDVS expression vector
[0062] Using the pVE5523-OMP16-PEDVS gene as a template, design the upstream primer F2 (see sequence SEQ.No.5) and downstream primer R2 (see sequence SEQ.No.6) for amplifying PEDVS,...
Embodiment 3
[0066] This example illustrates the comparison of the immune effects of OMP16-PEDVS lactic acid bacteria (the oral vaccine prepared in Example 1), OMP16-PEDVS protein and PEDVS protein (two injection vaccines prepared in Example 2).
[0067] 1. Animal immunity
[0068] Take 50 healthy female SPF grade (specific pathogen-free animals) C57 / B6 mice and divide them into OMP16-PEDVS lactic acid bacteria, OMP16-PEDVS protein group, OMP16-PEDVS protein+Freund's adjuvant group (OMP16-PEDVS+F' ), PEDVS protein group and control group. Those skilled in the art know that Freund's complete adjuvant generally plays a role in enhancing the immune effect in injection vaccines. The concentration used for protein immunization was 1 mg / ml, and the Freund's complete adjuvant group was added with protein:Freund's complete adjuvant for emulsification at a ratio of 1:1, and 200 μl of each mouse was injected into the leg muscles for immunization; 14 days after the first immunization, A booster imm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com