Method for removing ferric ion in water by using N element modified graphene electrode
A technology of graphene electrodes and iron ions, applied in separation methods, chemical instruments and methods, water/sewage treatment, etc., can solve problems affecting graphite performance, etc., and achieve the effect of simple operation, simple and easy preparation method, and short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of nitrogen-doped hydrogel material paper electrode includes the following steps:
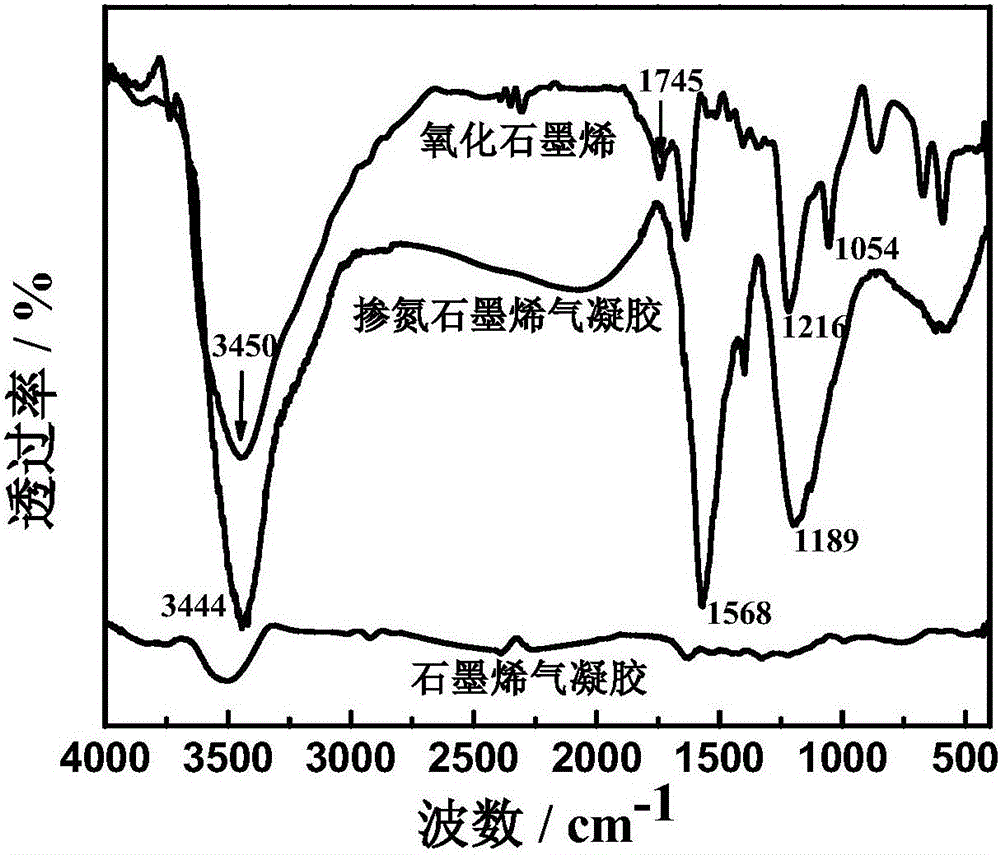
[0023] (1) Disperse 0.2 g of graphene oxide (GO) ultrasonically in 100 ml of distilled water, add 6 g of urea, and stir mechanically for 15 minutes. Pour the mixed solution into a hydrothermal reaction kettle and heat to 180°C for 12 hours. The resulting product was immersed in distilled water for 3 to 4 days, and finally the sample was freeze-dried at -50°C for 24 hours to obtain nitrogen-doped graphene aerogel.
[0024] (2) Add 90 mg of the nitrogen-doped graphene aerogel material prepared in step (1) to 2 mL of 4 wt% polyvinyl alcohol solution, and ultrasonically make the composite material uniformly dispersed in the solution. Take 0.16 mL of the above dispersion and evenly smear it on a 20mm×5mm hard paper sheet (thickness 400μm), freeze-dry and dry it at -50°C for 4h to make a nitrogen-doped graphene aerogel paper electrode.
[0025] The prepared nitrogen-doped graphene a...
Embodiment 2
[0027] The preparation process of the nitrogen-doped graphene aerogel paper electrode is the same as in the first embodiment.
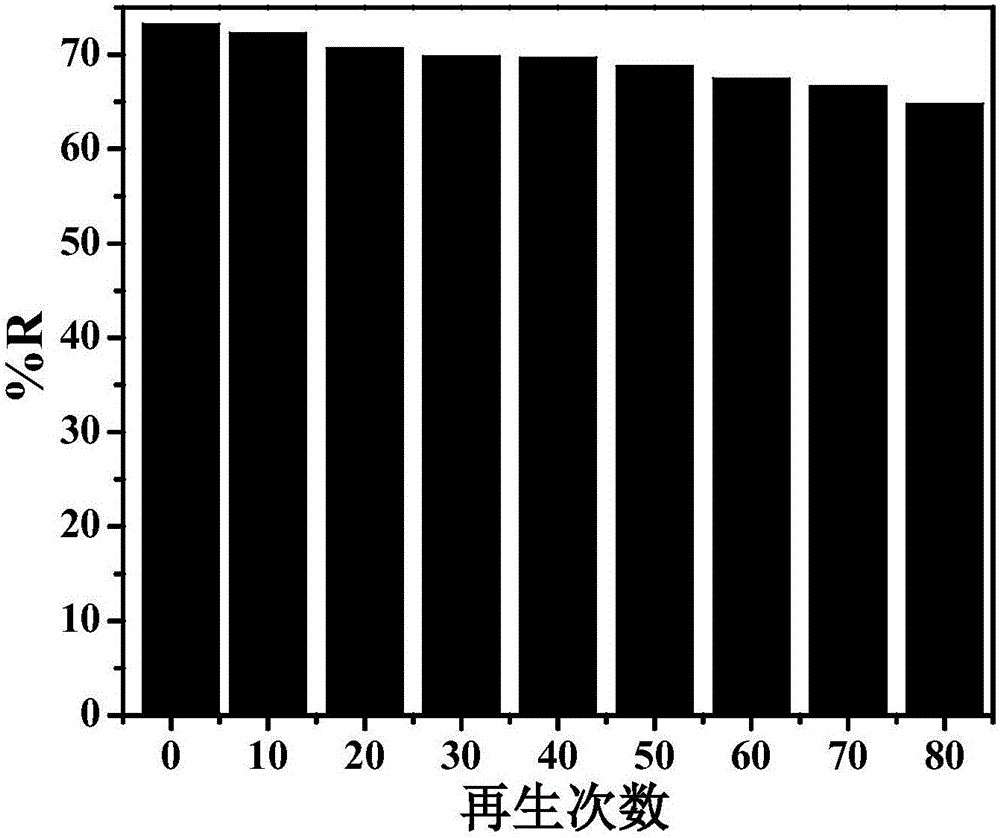
[0028] Cyclic electro-adsorption experiments were performed on nitrogen-doped graphene aerogel paper electrodes. Place the nitrogen-doped graphene aerogel paper electrode in 80 mL Fe with a concentration of 0.25 mmol / L 3+ In the solution, apply a potential of -0.3V, and record the conductivity of the solution. After 2 minutes, record the conductivity of the solution again to calculate the removal rate. Then the potential is removed to allow it to desorb, and the cycle continues for many times. The experimental results are as image 3 Shown. First adsorption of Fe 3+ The removal rate is 73.3%. After 80 cycles of use, the electrode pair Fe 3+ The removal rate is 64.8%, and the removal effect is slightly reduced.
Embodiment 3
[0030] The preparation process of the nitrogen-doped graphene aerogel paper electrode is the same as in the first embodiment.
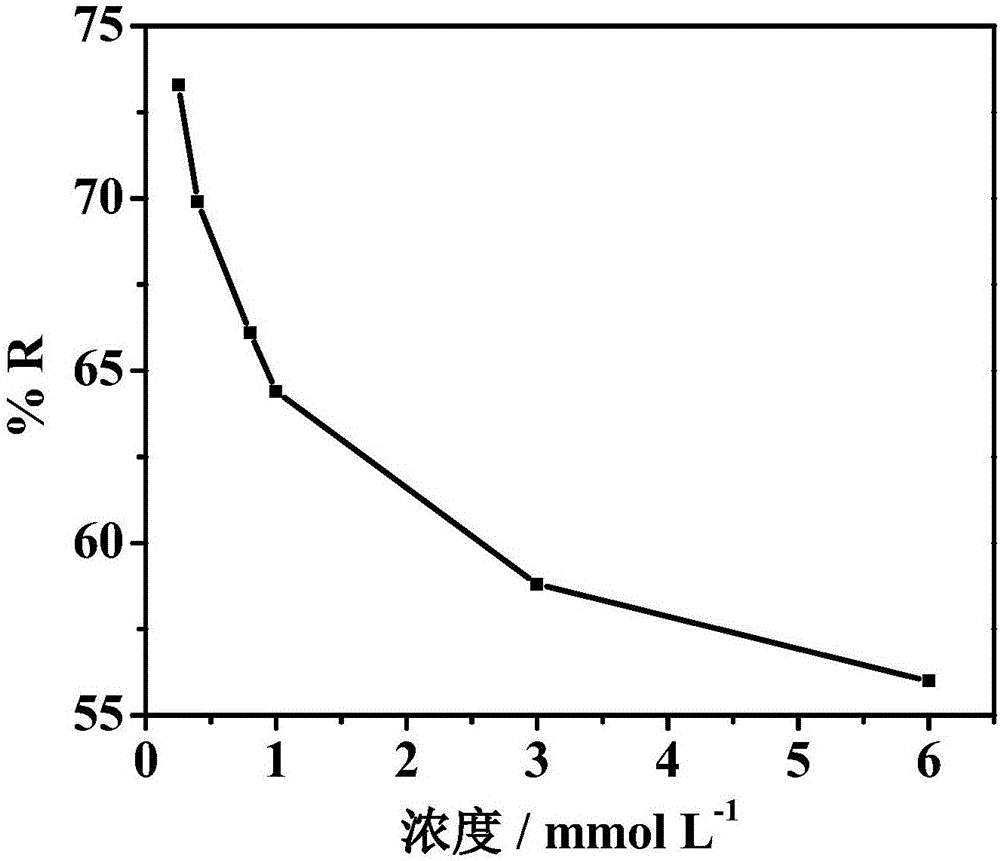
[0031] Nitrogen-doped graphene aerogel paper electrode prepared for 3mM Fe 3+ For electrochemical treatment of the solution, the applied voltage was -0.3V and the treatment time was 2 min. Such as Figure 4 As shown, the nitrogen-doped graphene aerogel paper electrode pairs Fe 3+ The removal rate is 58.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com