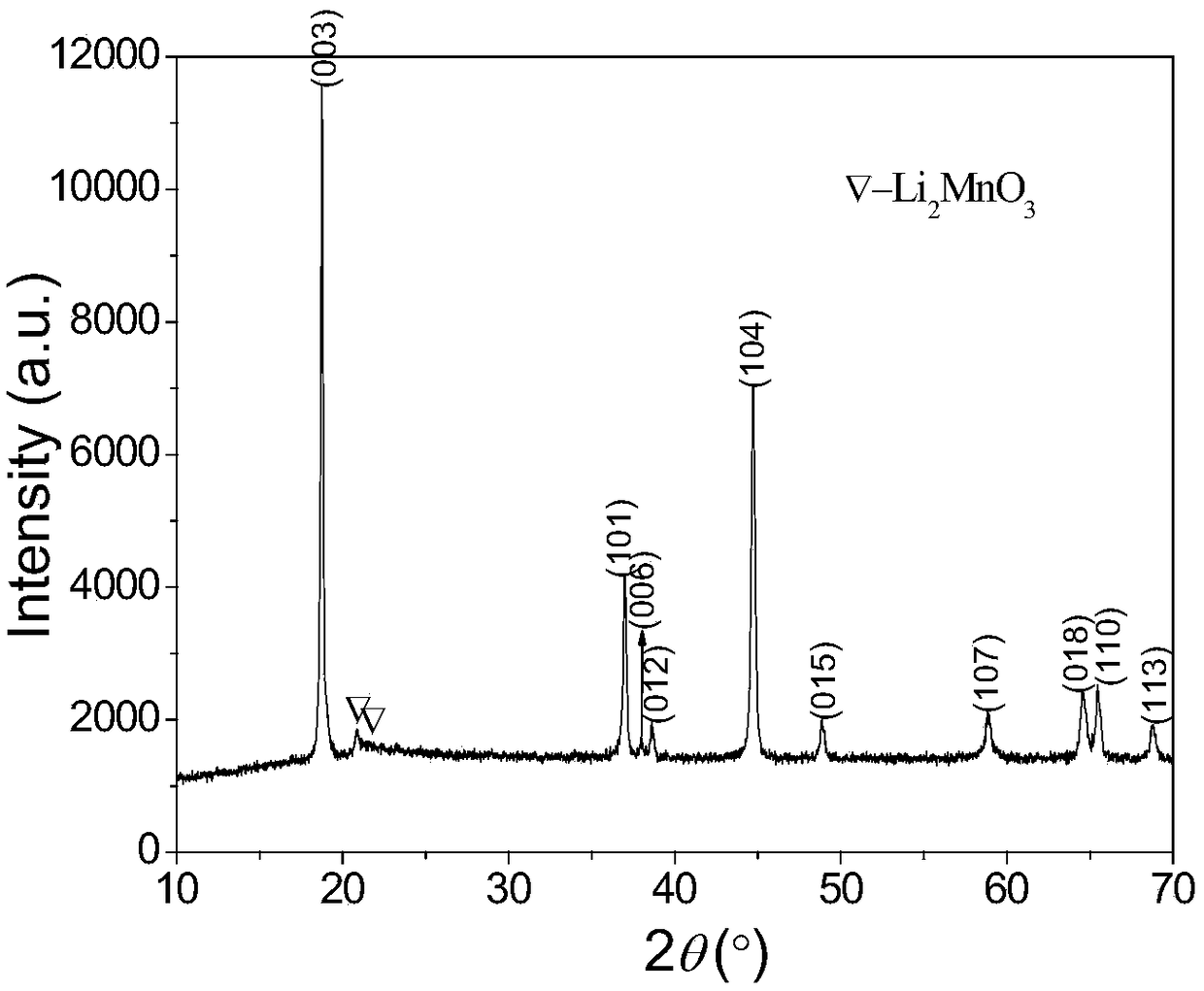
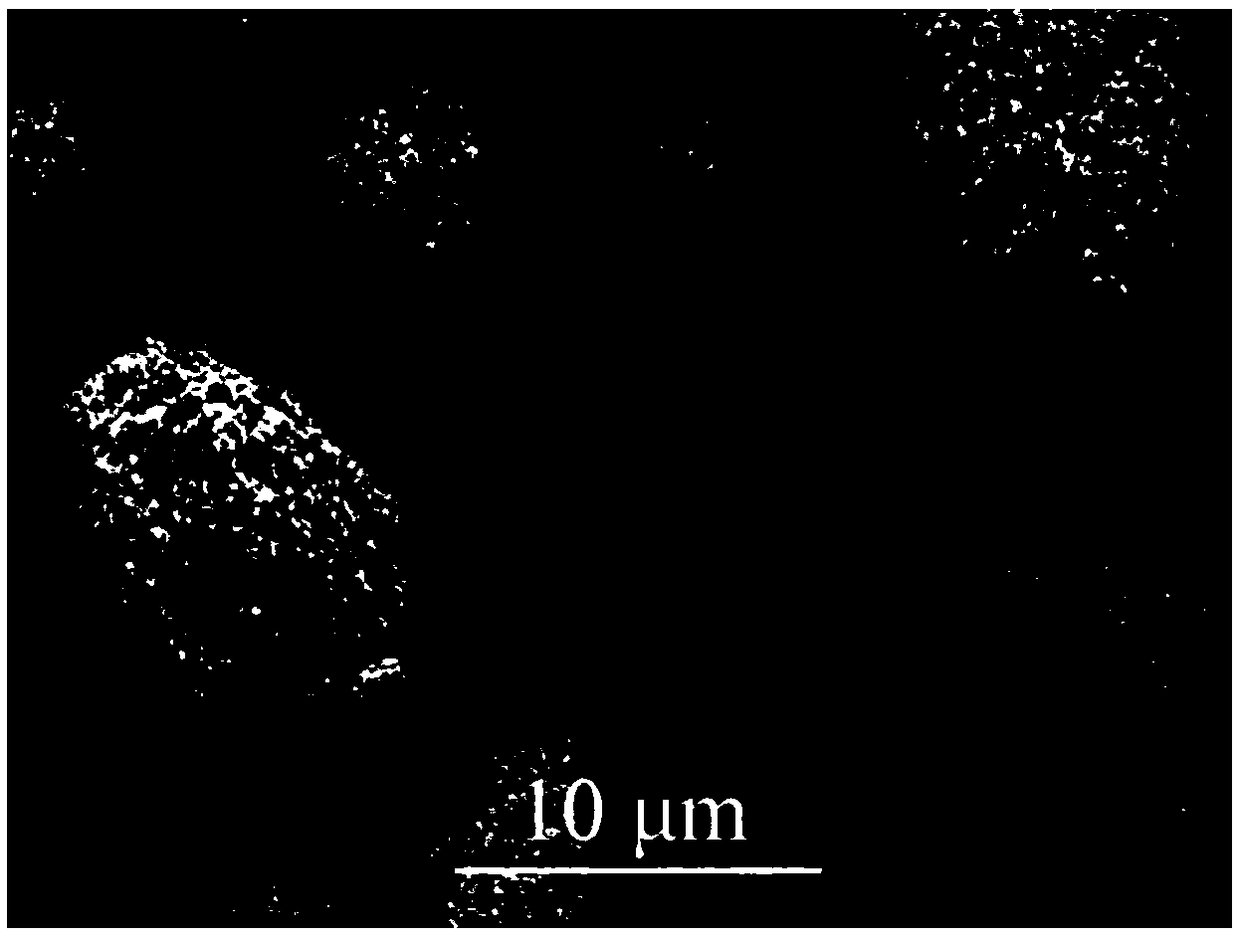
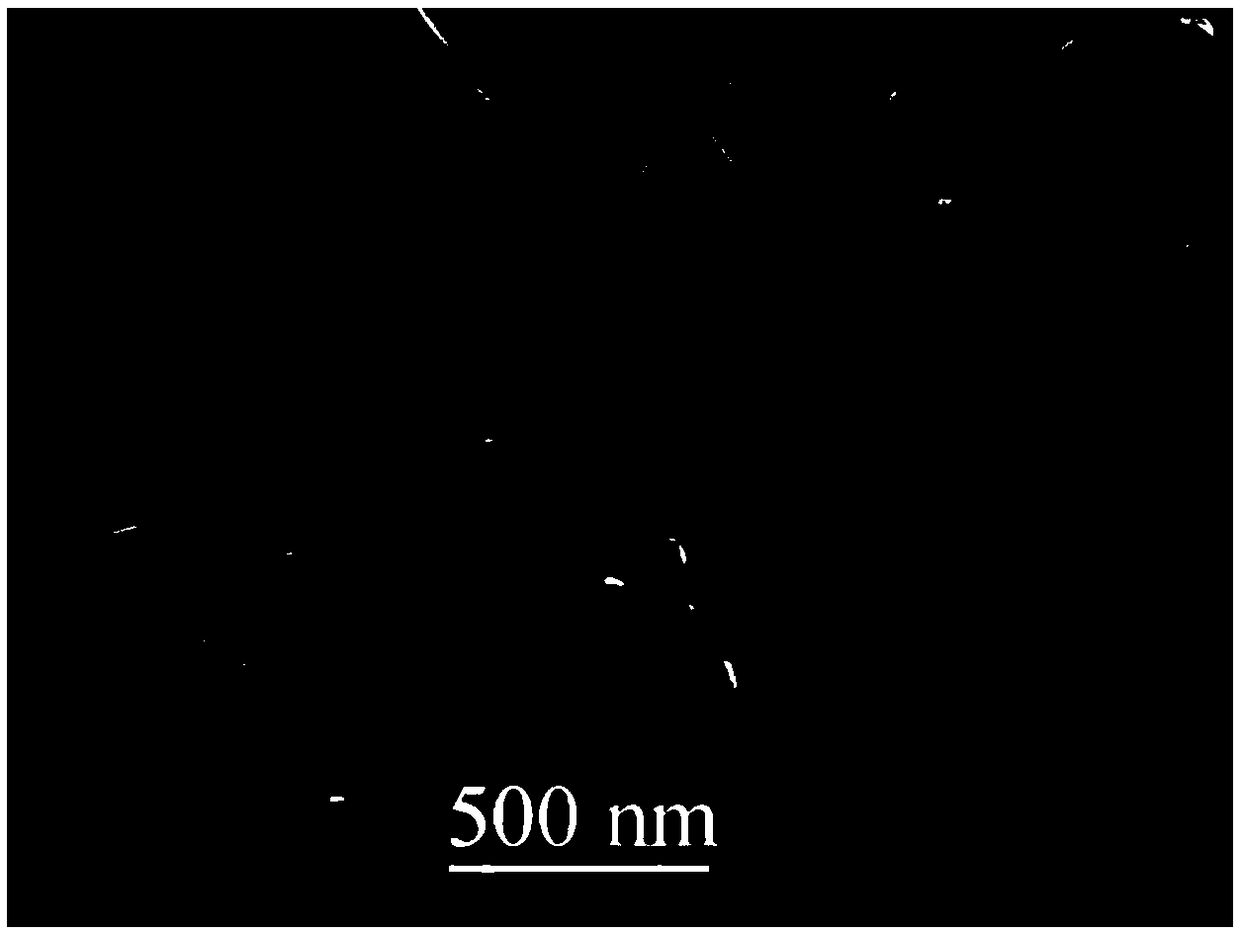
A kind of preparation method of layered lithium-rich manganese-based cathode material
A lithium-rich manganese-based, cathode material technology, used in battery electrodes, structural parts, electrical components, etc., can solve the problems of poor comprehensive electrochemical performance, unsatisfactory microscopic morphology, and low tap density of materials, and achieve cycle performance. Excellent, high tap density, high specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) According to the nominal chemical formula of layered lithium-rich manganese-based cathode materials 0.5Li 2 MnO 3 0.5LiMn 1 / 3 Ni 1 / 3 co 1 / 3 o 2 , weigh lithium hydroxide (LiOH·H 2 O) 3.3044g, manganese acetate (Mn(CH 3 COO) 2 4H 2 O) 8.1697g, nickel acetate (Ni(CH 3 COO) 2 4H 2 O) 2.0737g, cobalt acetate (Co(CH 3 COO) 2 4H 2 O) 2.0757g, oxalic acid (C 2 h 2 o 4 2H 2(2) 12.3943g, the transition metal ion acetate that weighs is dissolved in deionized water, is mixed with 250mL acetate solution, the oxalic acid that takes is dissolved in deionized water, is mixed with 350mL oxalic acid solution, will weigh Lithium hydroxide was added to the prepared oxalic acid solution, and continued to stir rapidly. After the lithium hydroxide was completely dissolved, the prepared acetate solution was added, and finally a small amount of deionized water was added to adjust the total volume of the reaction system to 645mL. The total concentration of metal ions is ...
Embodiment 2
[0032] (1) According to the nominal chemical formula 0.4Li of layered lithium-rich manganese-based cathode materials 2 MnO 3 0.6LiMn 0.5 Ni 0.5 o 2 , weigh lithium carbonate (Li 2 CO 3 ) 2.7155g, manganese acetate (Mn(CH 3 COO) 2 4H 2 O) 8.5782g, nickel acetate (Ni(CH 3 COO) 2 4H 2 O) 3.7326g, oxalic acid (C 2 h 2 o 4 2H 2 (2) 12.0302g, the transition metal ion acetate that takes by weighing is dissolved in deionized water, is mixed with the acetate solution of 350mL, the oxalic acid that takes by weighing is dissolved in deionized water, is mixed with the oxalic acid solution of 300mL, weighs The lithium carbonate that got was added in the prepared oxalic acid solution, and continued to stir rapidly. After the lithium carbonate was completely dissolved, the prepared acetate solution was added, and finally a small amount of deionized water was added to adjust the total volume of the reaction system to 750mL. The total concentration of metal ions is about 0.165mo...
Embodiment 3
[0038] (1) According to the nominal chemical formula of layered lithium-rich manganese-based cathode materials 0.6Li 2 MnO 3 0.4LiMn 0.3 Ni 0.3 co 0.4 o 2 , weigh lithium acetate (CH 3 COOLi·2H 2 O) 8.5697g, manganese acetate (Mn(CH 3 COO) 2 4H 2 O) 8.8232g, nickel acetate (Ni(CH 3 COO) 2 4H 2 O) 1.4930g, cobalt acetate (Co(CH 3 COO) 2 4H 2 O) 1.9932g, oxalic acid (C 2 h 2 o 4 2H 2 (2) 12.7583g, the transition metal ion acetate that weighs is dissolved in deionized water, is mixed with 250mL acetate solution, the oxalic acid that takes is dissolved in deionized water, is mixed with 300mL oxalic acid solution, will weigh Add lithium acetate to the prepared oxalic acid solution, and continue to stir rapidly. After the lithium acetate is completely dissolved, add the prepared acetate solution, and finally add a small amount of deionized water to adjust the total volume of the reaction system to 600mL. The total concentration is about 0.22mol / L;
[0039] (2) Mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com