A kind of oleuropein suppository
A technology of oleuropein suppositories and oleuropein, which is applied in suppository delivery, anti-inflammatory agents, non-central analgesics, etc. It can solve the problems of difficult control of inflammation, no oleuropein suppositories, bacterial resistance, etc., and achieve Prolonged action time, good lubricating effect, comfortable effect of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
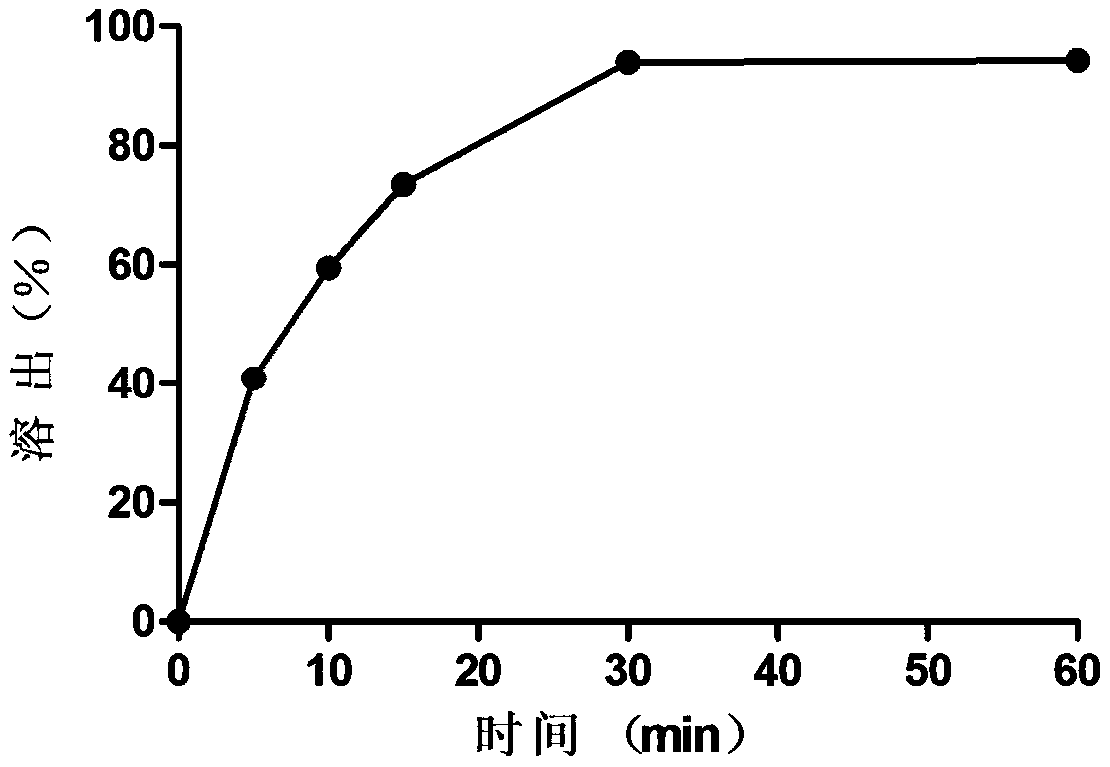
[0018] Example 1 Immediate Release Oleuropein Suppository
[0019] (1) Get carbomer 0.3g, add water 29.7g, leave standstill 4h, make the carbomer solution that concentration is 1%, namely hydrogel;
[0020] (2) Take oleuropein, grind it finely, weigh 3g after passing through a 100-mesh sieve, add 3g of oleuropein to the hydrogel several times, and stir evenly;
[0021] (3) Weigh 198g of PEG4000, heat and melt it at 80°C, and then keep it at 60°C;
[0022] (4) Weigh 22 g of the hydrogel mixed with oleuropein prepared in step (2), add it to the melted PEG4000, heat and stir for 20 minutes, and mix it evenly;
[0023] (5) Pour the matrix containing oleuropein obtained in step (4) into the suppository mold while it is hot, and make 100 quick-release oleuropein suppositories, each weighing 2.2 g, each containing 20 mg of oleuropein, It contains 2.18g of matrix, in which PEG4000 accounts for 90.83%, and hydrogel accounts for 9.17%.
[0024] Get 6 prepared oleuropein suppositories...
Embodiment 2
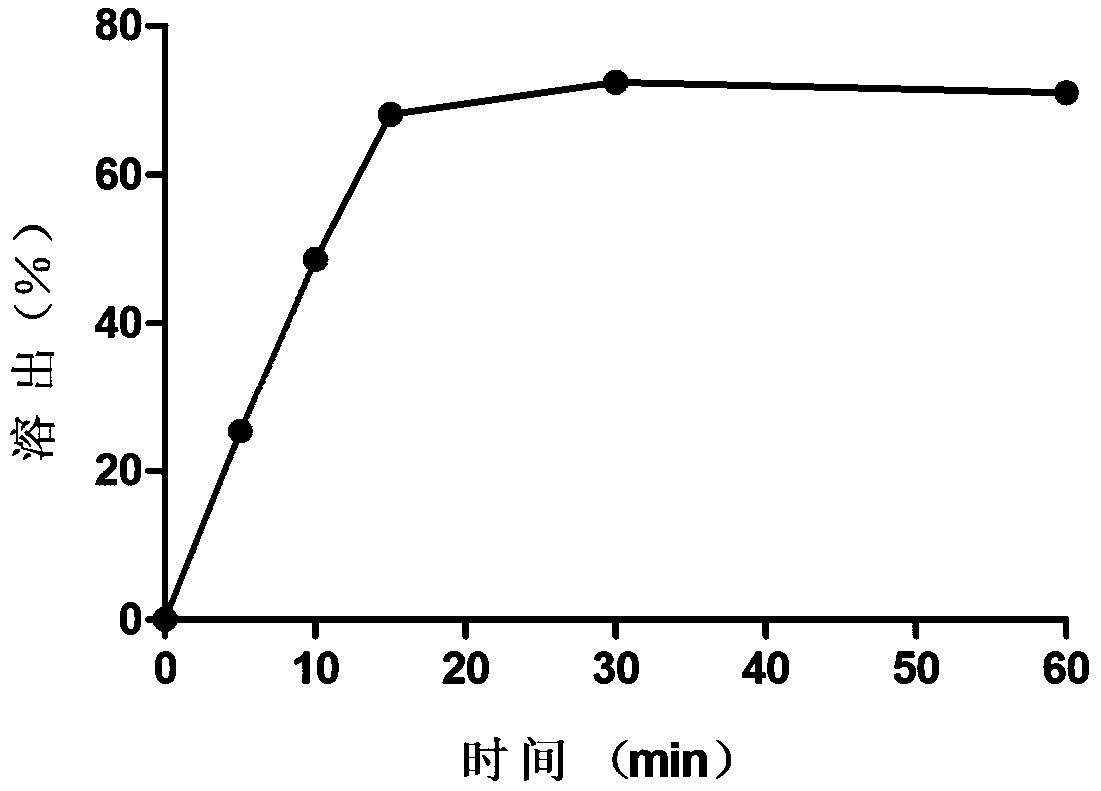
[0025] Example 2 Immediate Release Oleuropein Suppository
[0026] (1) Take 109g of PEG400, add 2g of oleuropein, ultrasonically or slightly heat to dissolve the oleuropein;
[0027] (2) Weigh 99g of PEG4000, heat and melt it at 80°C, and then keep it at 60°C;
[0028] (3) Add the oleuropein-containing PEG400 obtained in step (1) into the melted PEG4000 several times, stir while adding, and continue stirring for 30 minutes after adding;
[0029] (4) The matrix containing oleuropein obtained in step (3) was poured into the suppository mold while it was hot, and 100 quick-release oleuropein suppositories were obtained, each weighing 2.1 g, each containing 20 mg of oleuropein, It contains 2.08g of matrix, in which PEG4000 accounts for 47.60%, and PEG400 accounts for 52.4%.
[0030] Get 6 prepared oleuropein suppositories to measure the hardness, the average hardness is 1.70 ± 0.22kg; according to the second method of the dissolution and release determination method of the 2015 ...
Embodiment 3
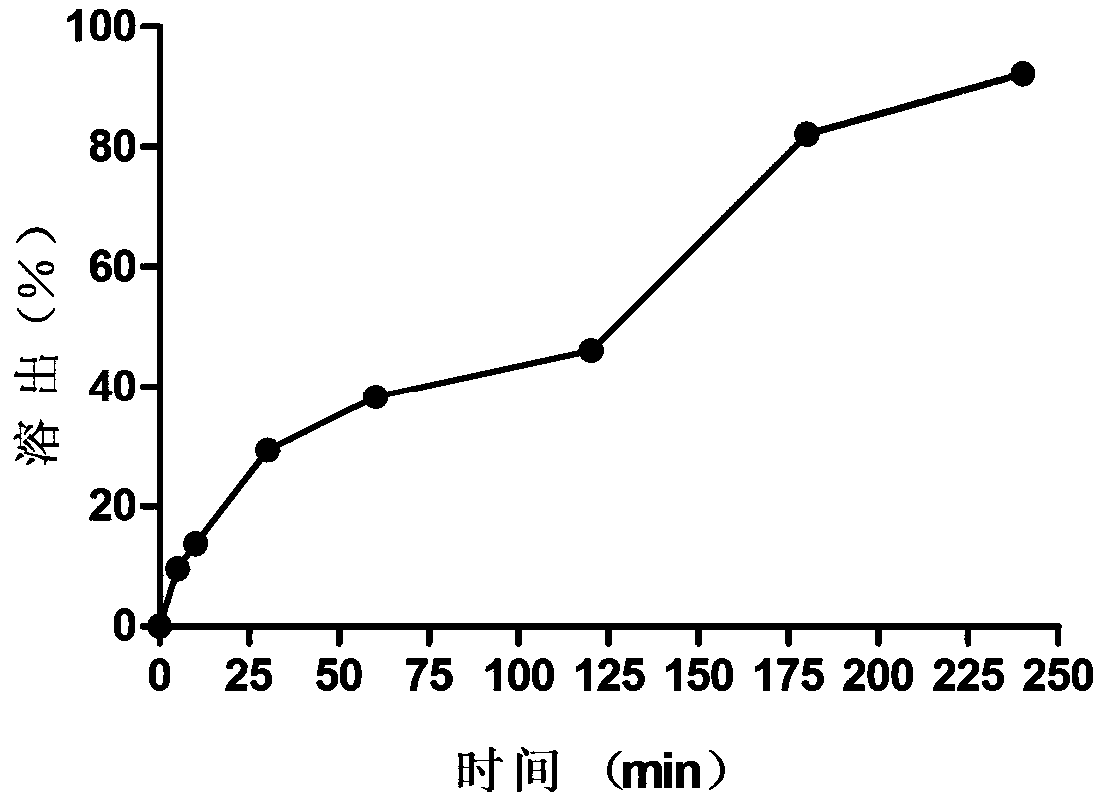
[0031] Example 3 Oleuropein Suppository with Moderate Release Rate
[0032] (1) Get 0.3 g of sodium carboxymethyl cellulose, add 29.7 g of water, let it stand for 4 hours, and prepare a sodium carboxymethyl cellulose solution with a concentration of 1%, namely hydrogel;
[0033] (2) Take oleuropein, grind it finely, weigh 3g after passing through a 100-mesh sieve, add 3g of oleuropein to the hydrogel several times, and stir evenly;
[0034] (3) Weigh 176g of PEG4000 and 22g of glyceryl monostearate, heat and melt them at 80°C, and then keep them warm at 60°C;
[0035] (4) Weigh 22 g of the hydrogel mixed with oleuropein prepared in step (2), add it to molten PEG4000 and glyceryl monostearate, heat and stir for 20 minutes, and mix it evenly;
[0036] (5) Pour the oleuropein-containing matrix obtained in step (4) into the suppository mold while it is hot to prepare 100 medium-release oleuropein suppositories, each weighing 2.2g, and each containing oleuropein 20mg , containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com