Paliperidone release rate progressive increasing preparation and preparation method thereof
A technology of paliperidone and release rate, which is applied in the field of preparation and preparation of paliperidone with increasing release rate, which can solve problems such as prominent adverse reactions, difficulty in reaching therapeutic concentration, complicated process, etc., and achieve blood drug concentration The effect of smooth curve, reducing the fluctuation of blood drug concentration and controlling the release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Coating the penetration enhancer I, the penetration enhancer II and the penetration enhancer III to obtain the coated penetration enhancer I, the coated penetration enhancer II and the coated penetration enhancer III with different weight gain.
[0046]
[0047] The HPMCE5 is hypromellose.
[0048]
[0049]
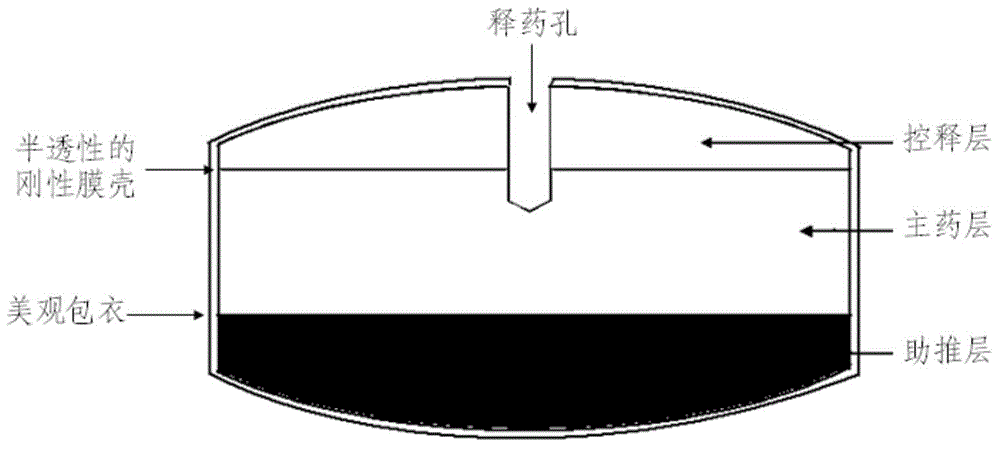
[0050] The drug structure of the present invention is as attached figure 1 shown.
Embodiment 2-10
[0051] The preparation technology of above-mentioned embodiment 2-10:
[0052] (1) Coating the penetration enhancer with an isolation material to obtain coated penetration enhancer I, coating penetration enhancer II and coating penetration enhancer III after coating isolation with different weight gain;
[0053] (2) Prepare controlled-release layer granules or powder after paliperidone, swelling material, penetration enhancer, binder and lubricant are uniformly mixed;
[0054] (3) Prepare the main drug layer granules or powder after uniformly mixing paliperidone, penetration enhancer, swelling material, coloring agent and lubricant;
[0055] (4) Mix the swelling material, coating penetration enhancer I, coating penetration enhancer II, coating penetration enhancer III, lubricant and coloring agent uniformly to prepare booster layer granules or powder;
[0056] (5) Press the above-mentioned prepared controlled-release layer, main drug layer and booster layer granules or powder...
Embodiment 11
[0060] In vitro cumulative release measurement of the preparation of paliperidone with increasing release rate prepared in Example 2:
[0061] Take this product, take 500ml of sodium chloride-hydrochloric acid solution with pH 1.2 as the dissolution medium, take 5ml of the solution respectively after 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 hours Centrifuge (with 5000 rpm centrifugal 5 minutes), get supernatant as need testing solution. Calculate the release amount of each tablet at different times.
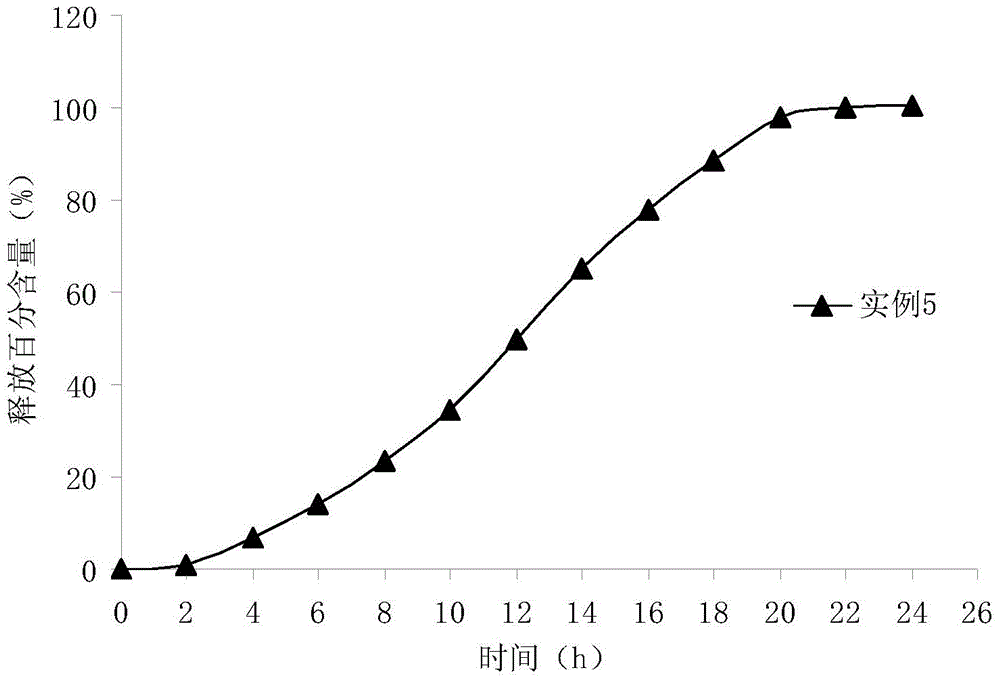
[0062] figure 2 Shown is the in vitro cumulative 24-hour release percentage-time curve of the preparation with increasing release rate of paliperidone prepared according to Example 6. The results show that the system has obvious drug release rate effect within 24 hours, and the drug release is complete, which is in line with clinical practice. need.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com