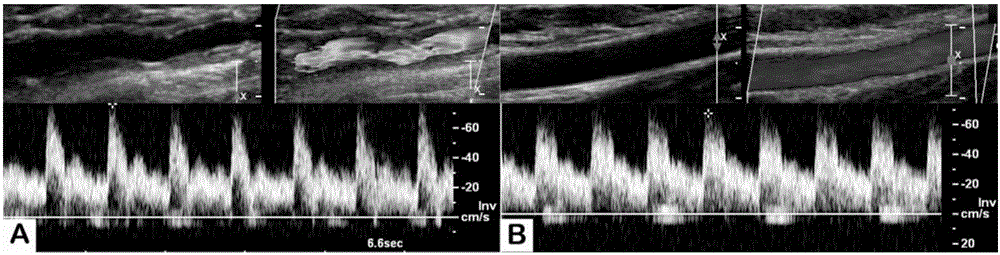
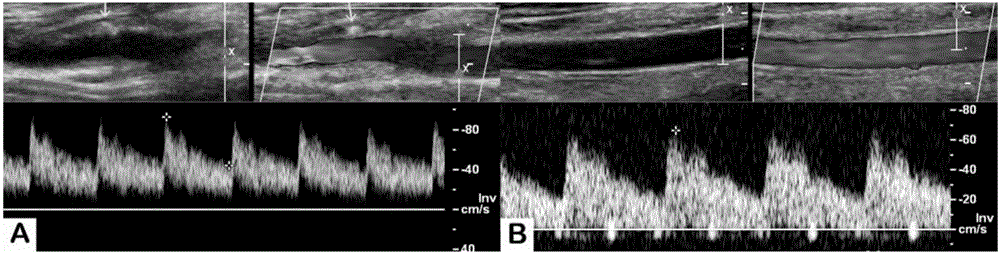
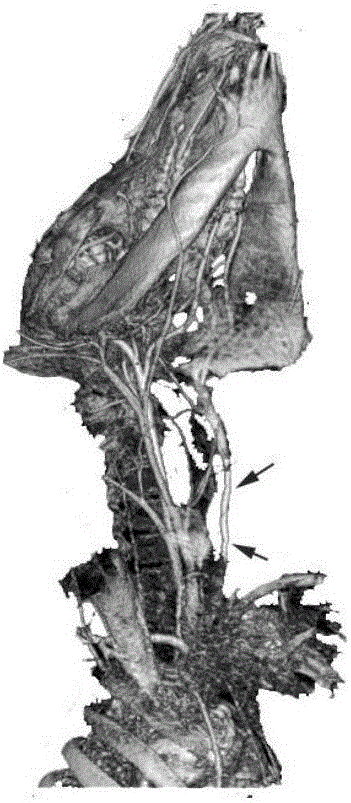
Artificial blood vessel and preparation method thereof
A technology for artificial blood vessels and tube walls, applied in the field of artificial blood vessels and its preparation, can solve the problems of poor mechanical properties of artificial blood vessels, low vascular patency, weak anticoagulant function, etc., and achieve good clinical application prospects, high patency, The effect of good anticoagulant function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The concrete steps of artificial blood vessel preparation method of the present invention are as follows:
[0048] (1) Preparation of autologous tissue pipeline
[0049] Cut the Teflon (Teflon) tube whose diameter matches the inner diameter of the blood vessel to be replaced to an appropriate length, sterilize it with 75% alcohol for 30 minutes, place it in the subcutaneous tissue, and take it out after 2 to 5 weeks, preferably 4 weeks. The surrounding newborn tissue was taken out, and the Teflon tube was extracted to obtain the autologous tissue pipeline. The thickness of the autologous tissue pipeline and the final artificial vascular product corresponding to 4 weeks are the most suitable, and the corresponding artificial vascular product has the best performance.
[0050] (2) Decellularization treatment
[0051] (2-1) Configuration of decellularization reagent: 3-[(3-cholamidopropyl)-diethylamine]-propanesulfonic acid (abbreviated as CHAPS reagent), EDTA.Na2, NaCl,...
Embodiment 1
[0064] (1) Preparation of autologous tissue pipeline
[0065] Cut a Teflon tube with a diameter matching the inner diameter of the blood vessel to be replaced to a suitable length, sterilize it with 75% alcohol for 30 minutes, and place it in the subcutaneous tissue. After 4 weeks, take out the subcutaneous Teflon tube together with the surrounding new tissue, and draw it out. In addition to the Teflon tube, an autologous tissue tube was obtained, the thickness of which was 324.1±57.4 microns (n=6, n is the number of test samples, and n has the same meaning below).
[0066] (2) Decellularization treatment
[0067] (2-1) Configuration of decellularization reagent
[0068]
[0069] According to the formula in the above table, add the accurately weighed reagent into a clean wide-mouth glass bottle, shake on the shaker for 2-3 hours to fully dissolve the reagent, at this time the solution becomes clear, prepare the decellularization reagent, and store it at 4°C for later use ...
Embodiment 2
[0083] (1) Preparation of autologous tissue pipeline
[0084] Cut the Teflon tube whose diameter matches the inner diameter of the blood vessel to be replaced to an appropriate length, sterilize it with 75% alcohol for 30 minutes, and place it in the subcutaneous tissue. After 2 weeks, take out the subcutaneous Teflon tube together with the surrounding new tissue, and draw it out. In addition to Teflon tubes, autologous tissue tubes were obtained with a thickness of 154.3±56.4 microns (n=6).
[0085] (2) Decellularization treatment
[0086] (2-1) Configuration of decellularization reagent
[0087]
[0088] According to the formula in the above table, add the accurately weighed reagent into a clean wide-mouth glass bottle, shake on the shaker for 2-3 hours to fully dissolve the reagent, at this time the solution becomes clear, prepare the decellularization reagent, and store it at 4°C for later use .
[0089] (2-2) Decellularization of autologous tissue pipeline
[0090]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com