Nine PTP1B inhibitors and synthesis method and application thereof
An inhibitor, chemical synthesis technology, used in oxime preparation, drug combination, carboxylate preparation and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
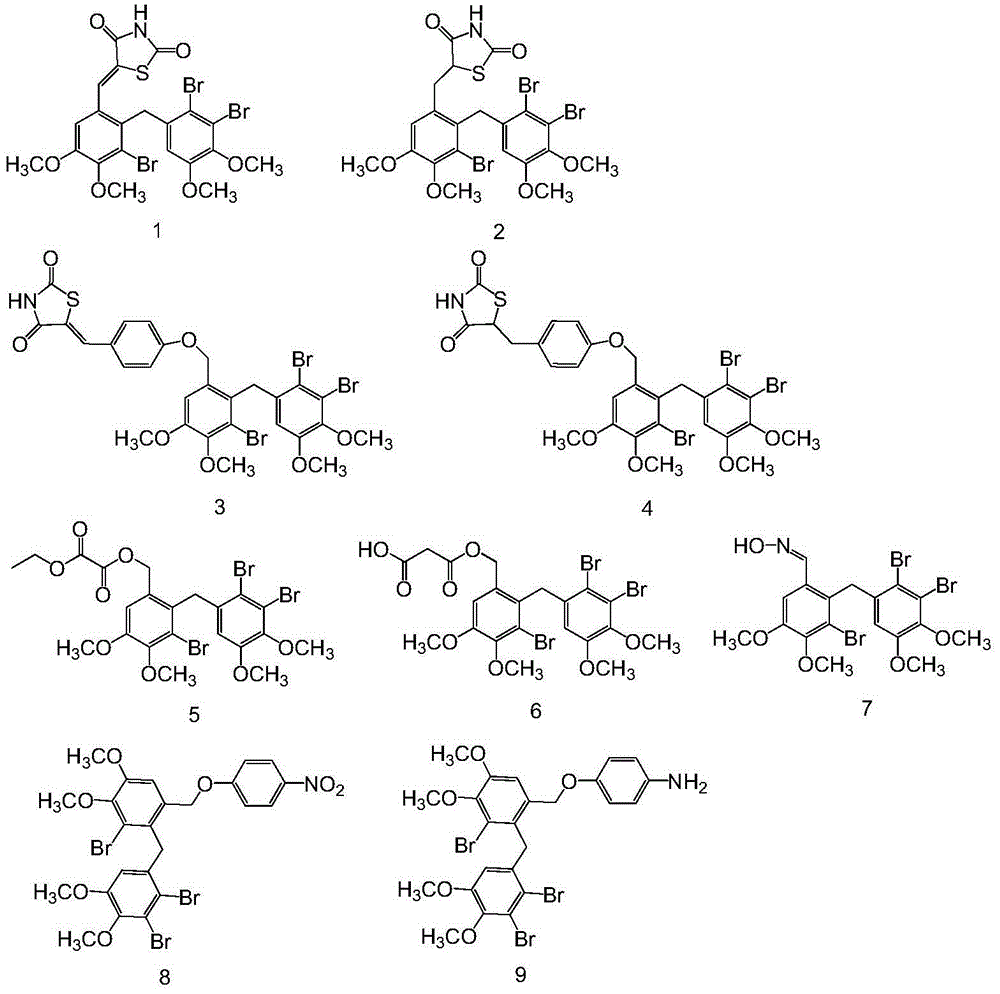
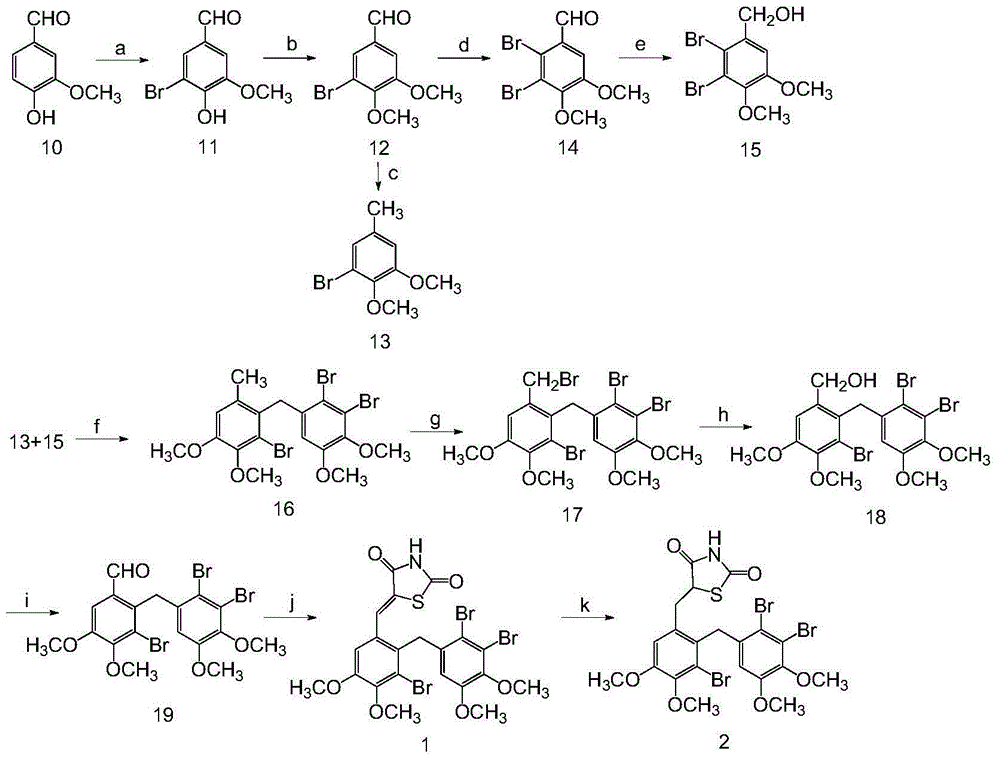
[0071] Example 1 "5-[3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzylidene]thiazolidine-2 , 4-diketone (1) and 5-[3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzyl]thiazole Total Chemical Synthesis and Structure Identification of Alkane-2,4-dione(2),"
[0072] (1) Chemical Synthesis and Structure Identification of 5-Bromovanillin
[0073] At 0°C, add 2.8mL of Br2 dropwise to 60mL of methanol with 7.6g (0.05mol) of vanillin dissolved therein, and finish adding in 2 hours, stir at room temperature for 1 hour, then add 25mL of water dropwise thereto at 0°C (with Precipitation), after 20 minutes of adding, continue to stir for 15 minutes, filter the precipitate, wash the precipitate with ice water, and drain to obtain 10.7 g of white crystals. After spectral analysis, it is confirmed that the compound is 5-bromovanillin;
[0074]The physical and chemical properties of the compound are as follows: white crystals, melting point 160-162°C; proton nuclea...
Embodiment 2
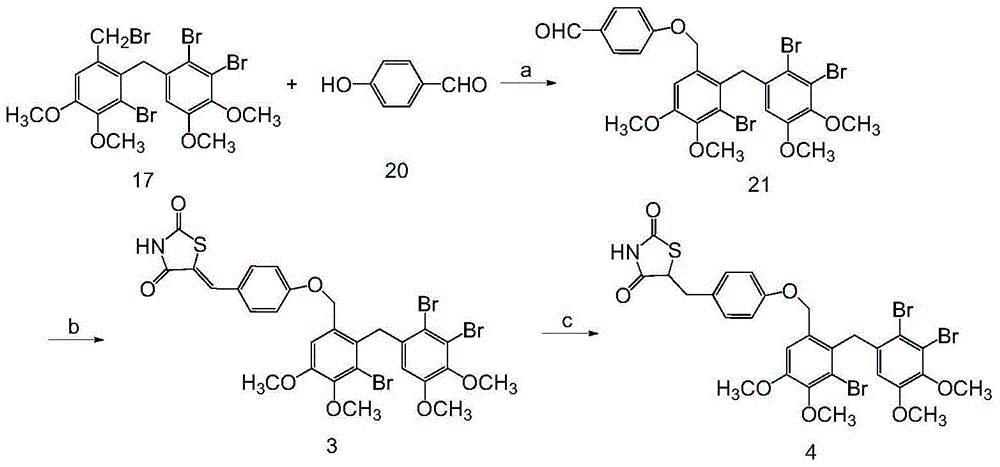
[0103] Example 2 "5-{4-[3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzyloxy]phenylene Methyl}thiazolidine-2,4-dione, 5-{4-[3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-di Chemical Total Synthesis and Structure Identification of Methoxybenzyloxy]phenyl}thiazolidine-2,4-dione
[0104] (1) 4-[3-bromo-2-(2′,3′-dibromo-4′,5′-dimethoxybenzyl)-4,5-dimethoxybenzyloxy]-benzene Chemical Synthesis and Structure Identification of Formaldehyde
[0105] Add 1.03 g of compound 17 to the reaction flask, dissolve in 10 mL of acetone, then add 0.306 g of p-hydroxybenzaldehyde and 0.462 g of K 2 CO 3 , TLC detection reaction process. After the reaction was completed, suction filtration was performed, and the filtrate was concentrated under reduced pressure. The oil was separated by column chromatography, and the eluent was petroleum ether: ethyl acetate = 6:1 to obtain 1 g of white solid.
[0106] The physical and chemical properties of the compound are as follows: white p...
Embodiment 3
[0113] Example 3 Chemical synthesis and structure of "3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzyl-ethyl oxalate" identification
[0114] (1) Synthesis and structure identification of 3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzyl-ethyl oxalate
[0115] Under ice bath conditions, 1 g of compound 18 was added to the reaction flask, dissolved in dichloromethane, then 119 mg of 4-dimethylaminopyridine (DMAP) was added, and 0.4 mL of ethyl oxalyl chloride was added. The progress of the reaction was detected by TLC. After the reaction was complete, the reaction solution was added into an equal volume of water and dichloromethane, and the organic phase was washed with saturated NaHCO3 and dried over anhydrous Na2SO4. The obtained oil was separated by column chromatography, and the eluent was petroleum ether: ethyl acetate = 4:1 to obtain 1.67 g of white solid.
[0116]The physical and chemical properties of the compound are as follows: white so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com