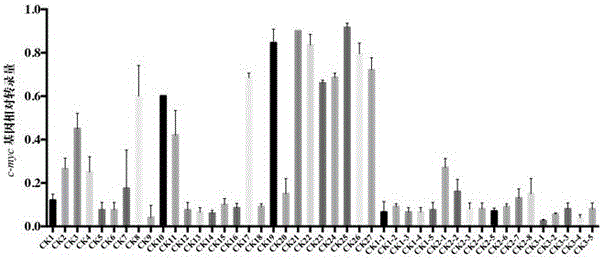
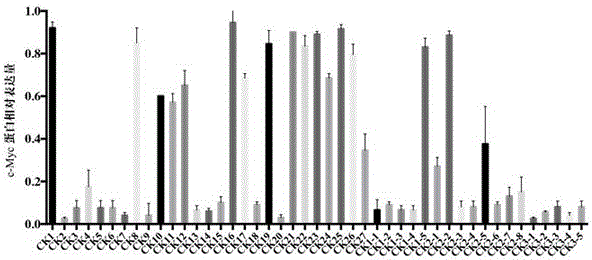
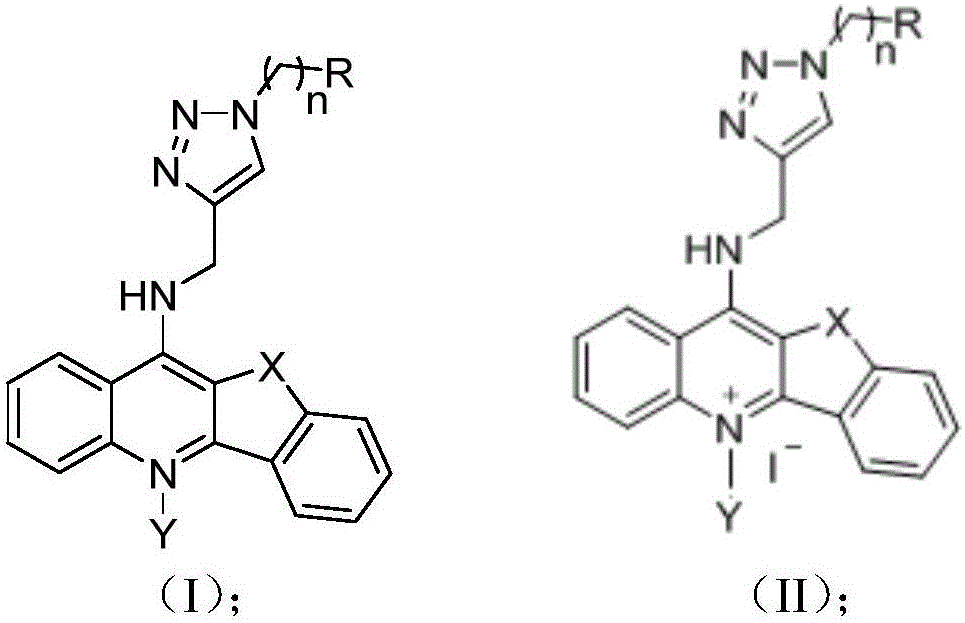
Quinolines derivative and preparation method thereof and application of quinolines derivative to preparation of anti-tumor medicine
A derivative and quinoline technology, applied in the field of medicinal chemistry, can solve the problems of limited resources, the need to improve the selection ability, and the limitation of anti-cancer applications, and achieve the effect of strong interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Synthesis of compound S4
[0055] Dissolve 0.1 mol of phenoxyacetic acid in 150 mL of chloroform, add 17.5 mL of thionyl chloride to reflux at 60°C for 3 hours, and then rotate under reduced pressure to remove the solvent to obtain a brown liquid. Then add acetonitrile as a solvent and mix with 0.1 mol of o-aminobenzene The formic acid undergoes a condensation reaction to obtain S1. The PPA is then preheated to 130° C. and added to S1 for cyclization reaction to obtain S2. S2 is reacted with thionyl chloride at 80°C under DMF catalysis to obtain compound S3. Then, 2.2 g of p-toluenesulfonic acid monohydrate was heated to 120° C. in a pressure tube, 5 mmol S3 was added, stirred for 10 minutes, and cooled to room temperature. Add 10mmol propargylamine and react at 120°C for 6 hours. After the reaction is completed, cool, dissolve the solid with 30mL chloroform: methanol=2:1, adjust the pH to 12 with 1N NaOH solution, then extract twice with 50mL chloroform, wash...
Embodiment 2
[0058] Example 2: Synthesis of compound CK1
[0059] Take compound S4 (0.25g, 1mmol) in a 50mL single-necked flask, add 10mL of tetrahydrofuran, add 2mL of pre-prepared solution containing (25mg, 0.1mmol) copper sulfate pentahydrate, 2mL of the ready-to-use containing (50mg, 0.2 mmol) sodium ascorbate solution, and finally add 1.2 equivalents of: N,N-dimethyl-2-azidoethylamine. The reaction was stirred at 35°C and monitored by TLC. After the reaction is completed, the solvent is spin-dried and purified by column chromatography to obtain a pale yellow solid CK1 (mobile phase: dichloromethane: methanol = 200-50:1; ammonia 0.5%).
[0060] The yield was 88%. m.p.121.5-122.6℃; 1 H NMR(400MHz, CDCl 3 )δ8.41(d,J=7.7Hz,1H), 8.18(dd,J=8.5,0.7Hz,1H),7.94(d,J=8.5Hz,1H),7.71(s,1H),7.66– 7.58(m,3H),7.43(ddd,J=15.3,7.1,1.2Hz,2H),5.94(s,1H),5.36(d,J=5.8Hz,2H), 4.38(t,J=6.2Hz ,2H), 2.69(t,J=6.2Hz,2H), 2.19–2.14(s,6H). 13 C NMR(101MHz, CDCl 3 )δ158.1,147.1,146.9,145.5,133.7,130.0,129.5,128.0,12...
Embodiment 3
[0062] Example 3: Synthesis of compound CK2
[0063] The method is the same as in Example 2, except that N,N-diethyl-2-azidoethylamine is used instead of N,N-dimethyl-2-azidoethylamine to obtain CK2 as a light yellow solid.
[0064] The yield was 83%. m.p.133.1-134.3℃; 1 H NMR(400MHz, CDCl 3 )δ8.39(d,J=6.9Hz,1H), 8.19(d,J=8.4Hz,1H),7.93(d,J=8.5Hz,1H), 7.63(dt,J=19.1,7.5Hz, 4H),7.45(t,J=7.3Hz,2H),5.81(s,1H),5.36(d,J=5.8Hz,2H),4.33(s,2H),2.78(s,2H), 2.40( d, J = 6.9 Hz, 4H), 0.79 (t, J = 6.9 Hz, 6H). 13 C NMR(101MHz, CDCl 3 )δ158.1,146.9,146.7,145.3,133.8,133.7,130.1,129.4,128.1,124.1,123.3,123.2,122.8,122.2,120.4,118.1,111.8,52.7,49.0,47.1,40.9,11.7. HPLC purity: 99.2% .HRMS(ESI)m / z:calcd forC 24 H 26 N 6 O,[M+H] + ,415.2241found 415.2246.
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com