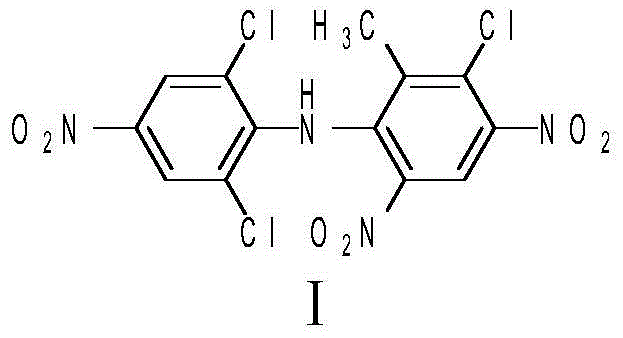
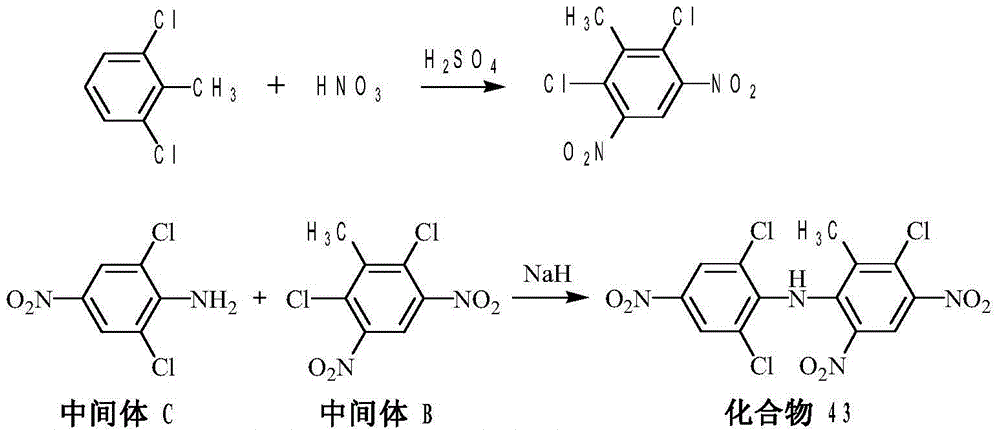
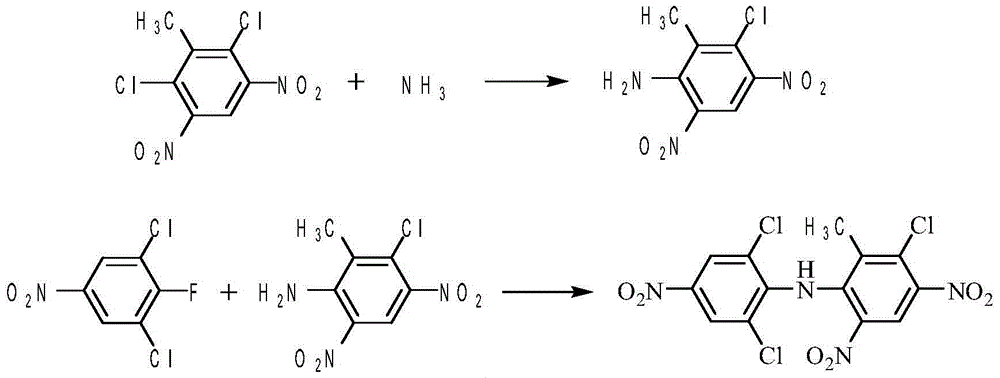
Method of preparing SYP-14288
A technology of bibendenil and dinitrotoluene, which is applied in the field of preparation of bibendenil, can solve the problems of poor product purity, low reaction yield, and generation of impurities, and achieve improved yield and content, broad market prospects, The effect of good competitive advantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 126g (0.5mol) of 2,6-dichloro-3,5-dinitrotoluene into a 500mL reaction flask, then add 250mL of DMF, raise the temperature to 50°C, and feed about 13.0g of ammonia gas within 2 hours ( 0.75mol), after the gas is passed through, keep it warm for 1 hour and take samples for analysis. After the reaction was analyzed by HPLC, the reaction was distilled under reduced pressure, and the solvent was removed to 100° C. at 0.09 MPa until no obvious fraction was separated. Approximately 200 mL of DMF was removed. Stop the precipitation, lower the temperature, drop the temperature in the container to 80°C, add 300mL of water to the reaction flask, stir at 50°C for 1 hour, cool down to room temperature, filter, wash with 60mL of water, and dry to obtain 108.4g of substituted amides, with a yield of 89.1 %, purity 95.1%.
[0042]Put 116g (0.5mol) of the substituted amides obtained in the above manner, 126g (0.6mol) of 3,5-dichloro-4-fluoronitrobenzene, 138g (1.0mol) of potassiu...
Embodiment 2
[0044] Add 48kg of 2,6-dichloro-3,5-dinitrotoluene and 100kg of DMF into the 300L reaction kettle, and pass 6.5kg (0.38kmol) of dry ammonia gas into the reaction flask within 3 hours while stirring. This reaction is an exothermic reaction, and the reaction temperature is 20°C, the highest not exceeding 40°C. Then the reaction was continued for 2 hours, and the reaction progress was tracked by liquid chromatography. After the reaction was complete, air was bubbled through for 1 hour to drive off excess ammonia gas.
[0045] The solvent is evaporated under reduced pressure, the vacuum is 0.095MPa, and the distillation is finished when the recovery rate of DMF is about 80%. After evaporating the solvent, slowly add 110L of water at 80°C, not too fast, otherwise the temperature will drop too quickly and a large solid will easily form, then stir at 60°C for 0.5 hour. Cool down to 20°C, filter, wash with 20 L of water, and dry to obtain 43 kg of substituted amides with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com