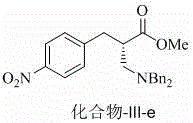
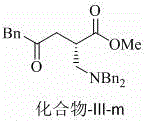
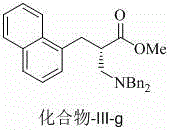
Chiral beta 2-amino acid derivative and preparing method thereof
A technology for amino acids and derivatives, which is applied in the field of chiral β2-amino acid derivatives and their preparation, can solve the problems of general chiral control, structural diversity and limited derivatives, and achieve high e.r value and total yield. The effect of simple processing and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 1) Preparation of mixed anhydride (Compound-I):
[0034] Compound-I is composed of acid (compound-V) and pivaloyl chloride, dichloromethane is used as the reaction solvent, the reaction concentration is 0.2-1M, triethylamine is added, and the reaction temperature is 0-30 o C, the mixed acid anhydride prepared in situ is directly used in the reaction without separation. In the preparation of compound-I, the molar ratio of compound-V, pivaloyl chloride and triethylamine is 1:1-3:1-3, preferably 1:1:1.2, and the preferred reaction temperature is 0 o C, the reaction concentration is 0.3M, and the reaction time is 2 hours.
[0035] 2) Preparation of protected benzylamine (compound-II):
[0036] Reference Angew. Chem. 1996, 108, 1059 prepared.
[0037] 3) Preparation of Compound-III:
[0038] Add azacarbene catalyst precursor (NHC-I) to the mixed anhydride (compound-I) prepared in situ to protect benzylamine (compound-II), and the reaction temperature is 0-40 o C. Stir u...
Embodiment 1
[0042] According to the following preparation route:
[0043]
[0044] 1) Add phenylpropionic acid (Compound-V-a) (3.6g, 24.0mmol) dissolved in dry dichloromethane (80ml) to a 250ml round bottom flask under nitrogen protection under ice-water bath, triethylamine ( 4.0ml, 28.8mmol), slowly added pivaloyl chloride (2.95ml, 24.0mmol) dropwise, and kept stirring at this temperature for 2 hours to prepare a mixed anhydride solution. The prepared mixed anhydride is directly used in the carbene catalyzed reaction.
[0045] 2) At room temperature, continue to add protected benzylamine (compound-II) (4.83g, 20mmol) and azacarbene catalyst precursor (NHC-I) (838mg, 2.0mmol), heating and maintaining the reaction temperature at 35 o C, the reaction time is 24 hours. The reaction solvent was spin-dried, followed by flash column chromatography (ethyl acetate:petroleum ether=1:10), and white solid compound-III-a was obtained, 5.22g, with a yield of 70%. 1 H NMR (400 MHz, CDCl 3 ) δ 7...
Embodiment 2
[0048] According to the method of Example 1, compound III-b was prepared:
[0049]
[0050] White solid, yield 70%. 1H NMR (400 MHz, CDCl 3 ) δ 7.29-7.22 (m, 10H), 7.03 (d, J = 8.0 Hz, 2H), 6.95 (d, J = 8.0 Hz, 2H), 3.66 (d, J = 13.6 Hz, 2H), 3.56(s, 3H), 3.42 (d, J = 13.6 Hz, 2H), 3.00-2.93 (m, 1H), 2.81 (dd, J 1 = 12.8Hz, J 2 = 9.2 Hz, 1H), 2.71 (d, J = 7.6 Hz, 2H), 2.51 (dd, J 1 = 12.8 Hz, J 2 =6.0 Hz, 1H), 2.29 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ 175.0, 139.1, 136.1, 135.7, 129.1, 129.0, 128.5, 128.1, 126.9, 58.4, 55.8, 51.4, 46.9, 36.0, 21.0; HRMS (ESI, m / z): H calcd. for C26H29NO2 + 388.2277, found 388.2278. [α] 21 D = +21.4 (c = 1.0 in CHCl 3 ); HPLC analysis: 95:5er.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com