Method for recovery of d-alpha-tocopherol from natural vitamin E leftover material
A technology of natural vitamins and tocopherols, applied in organic chemistry and other directions, can solve the problems of large consumption of fillers and solvents, high separation costs, and complex processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
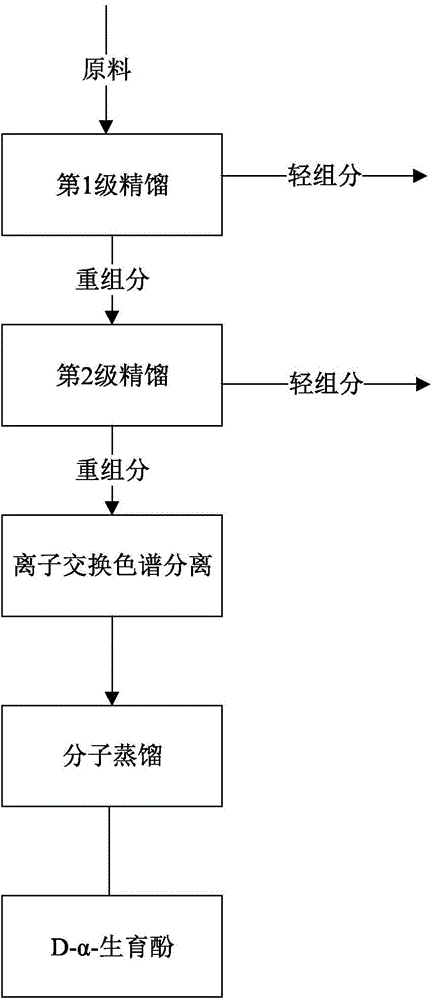
[0025] Embodiment 1: the 1st stage rectification step (1.1) of scrap
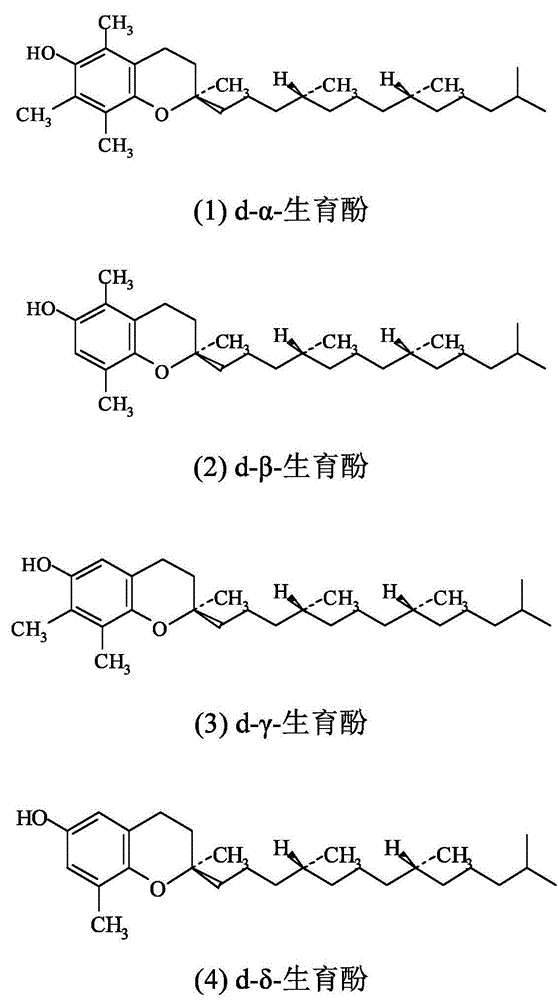
[0026] Such as figure 1 As mentioned above, 500 g of scraps (derived from untransformed d-α-tocopherol during the extraction process of amine methylation reaction, specifically refer to Chinese patent CN102633766) were added to a 1L round-bottomed Erlenmeyer flask, wherein the total tocopherol content was 10.2 %, d-alpha-tocopherol relative to 97.5%. A glass rectification tower with an inner diameter of 2.6mm and a height of 700mm is used. The tower is filled with 30mm*30mm stainless steel θ rings. The tower is equipped with a silver-plated jacket and an electric heating jacket for heat preservation. The transformer controls the heating power of the heating jacket and the reflux ratio controller. Carry out rectification under the vacuum degree of 20-40Pa at the top of the tower. The kettle temperature was 200°C, the tower top temperature was 160°C, and the appropriate reflux ratio was adjusted to distill ...
Embodiment 2
[0029] Embodiment 2: the 1st stage rectification step (1.1) of scrap
[0030] Such as figure 1 Said, in a 1L round-bottomed Erlenmeyer flask, add scraps (derived from untransformed d-α-tocopherol in the extraction process of amine methylation reaction, see Chinese patent CN102633766) (500g, wherein the total tocopherol content is 15.8% , d-α-tocopherol is relatively 96.2%.Use a glass rectification tower with an inner diameter of 2.6mm and a height of 500mm. The tower is filled with a 30mm*30mm stainless steel θ ring. The tower is equipped with a silver-plated jacket and an electric heating jacket for heat preservation. Transformer control Heating mantle heating power, reflux ratio controller. Rectification is carried out under the vacuum degree of 20-40Pa at the top of the tower. The temperature of the kettle is 190°C, and the temperature of the top of the tower is 160°C. Adjust the appropriate reflux ratio to distill 286g of light components, and the remaining 207g Heavy compo...
Embodiment 5
[0037] Embodiment 5: the 2nd stage rectification step (1.2) of scrap
[0038] Such as figure 1 As described above, 300g of the heavy component in Example 1 is introduced into the second-stage rectification tower, wherein the inner diameter of the tower is 2.6mm, the height of the tower is 350mm, and the tower is filled with a 30mm*30mm stainless steel θ ring, and the outer belt of the tower is silver-plated. The electric heating jacket is kept warm, the transformer controls the heating power of the heating jacket, and the reflux ratio controller. Under the vacuum condition of 10-20Pa, the kettle temperature is 240°C, and the tower top temperature is 210°C, most of the squalene is distilled out. 128 g of light components were distilled off, 167 g of heavy components remained, 5 g was lost. The total tocopherol content and yield in each component are shown in Table 5 (test results of Example 5);
[0039] table 5
[0040] components mass (g) Total Tocopherol Con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com