Dipeptide hydrogel, and preparation method and application thereof
A hydrogel, selected technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve simple preparation methods, good self-healing properties, and good shear thinning properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Pre-dissolve 2mg of N-fluorenylmethoxycarbonyl-protected tyrosine-aspartic acid dipeptide (Fmoc-YD) in 1uL of hexafluoroisopropanol (HFIP), then add 500uL of water to the system, and place at 40kHz Under ultrasonication for 0.5 minutes, the above mixed solution was aged at 25°C for 24 hours, and Fmoc-YD hydrogel was finally obtained.
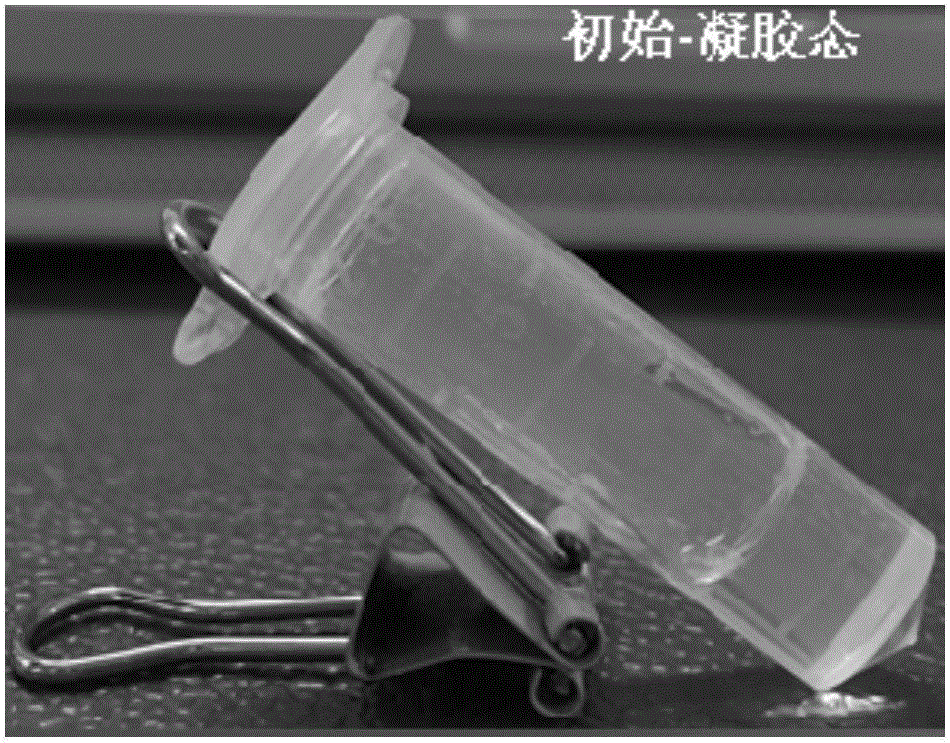
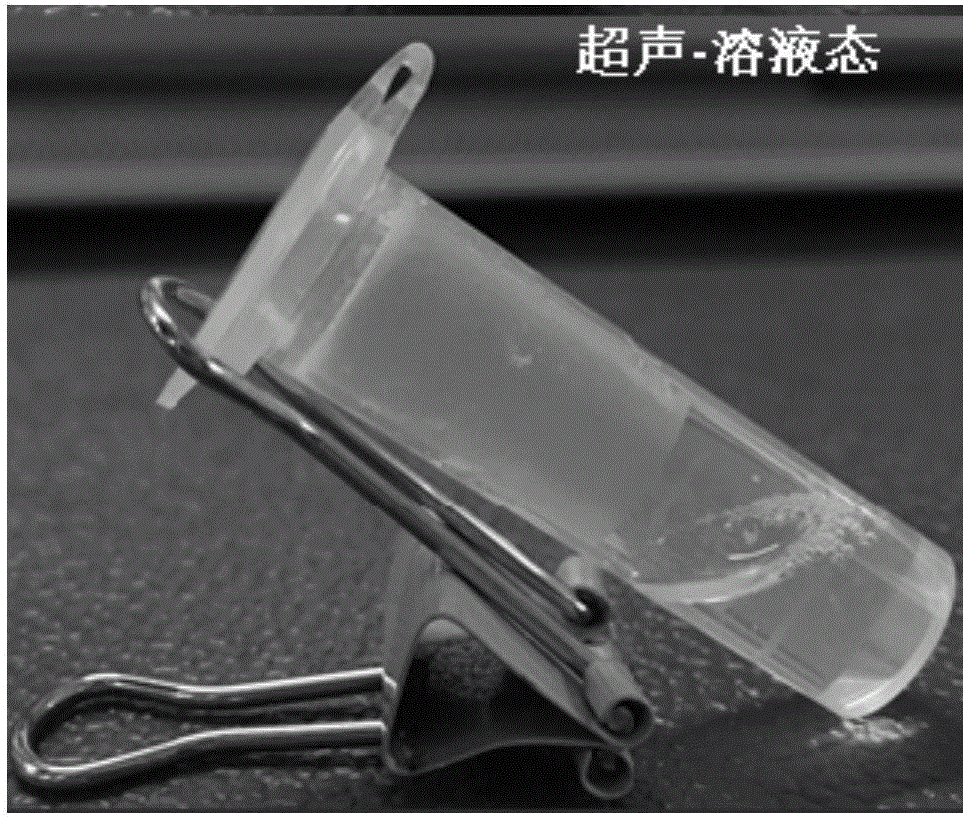
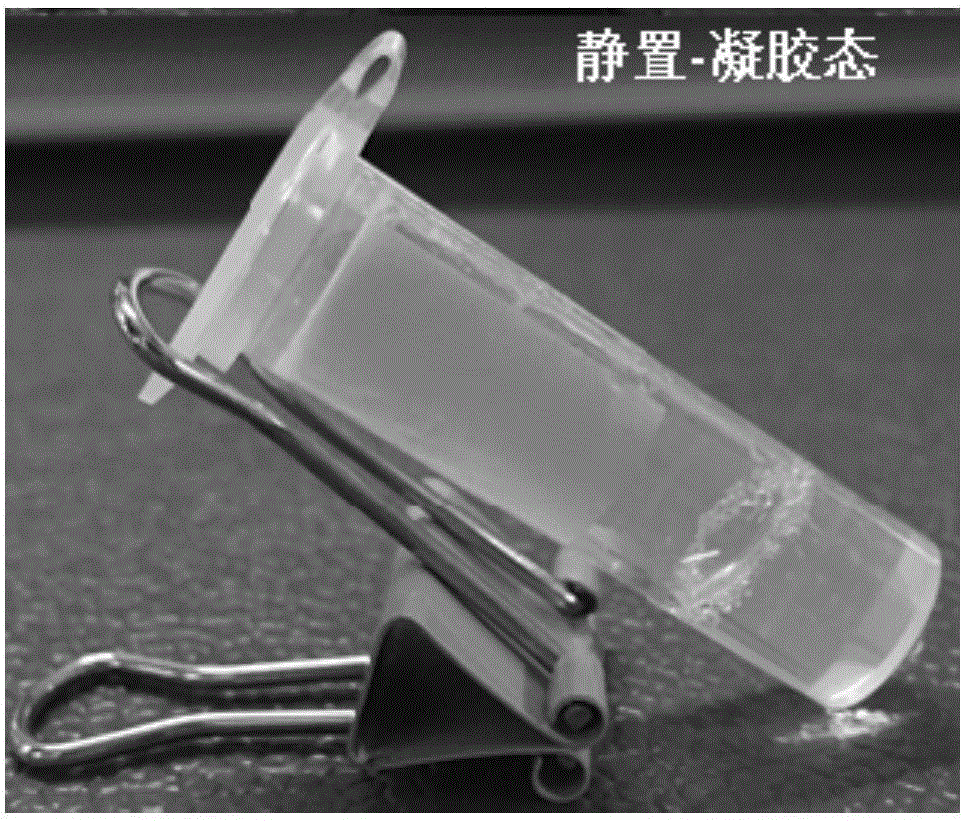
[0052] The physical picture of the obtained hydrogel is shown in Figure 1(a). The gel does not flow after being tilted, and the appearance is uniform and transparent. The gel has good ultrasonic responsiveness. After ultrasonication, the gel changes from a gel state to a sol state, and can quickly self-heal in a short time and return to the gel state, such as Figure 1(b) and 1(c) .
Embodiment 2
[0054] Pre-dissolve 4 mg of phenylalanine-aspartic acid (Azo-FD) containing phenyl (Azo-) dipeptide in 5 uL dimethyl sulfoxide (DMSO) solution, add to the DMSO solution of Azo-FD 500uL of water was mixed evenly, and the above mixed solution was ultrasonically placed at 20kHz for 1 minute, and the solution was taken out and aged at 37°C for 48 hours. Finally, Azo-FD hydrogel was obtained.
[0055] Inject the hydrogel onto the glass slide with a syringe as figure 2 , the resulting hydrogel has shear-thinning properties and can be injected.
Embodiment 3
[0057] Weigh 3mg of indole-protected tryptophan-glutamic acid dipeptide derivative Indo-WE and dissolve it in 5uL of dimethylformamide (DMF), then add 1mL of water to the DMF and mix well, put the above solution in After ultrasonication at 35kHz for 2 minutes, take it out, and leave it at 30°C for aging reaction for 72 hours. A dipeptide hydrogel based on Indo-WE was obtained.
[0058] The atomic force picture of the obtained hydrogel is as follows image 3 , the obtained hydrogel is a three-dimensional network structure composed of interwoven fibers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com