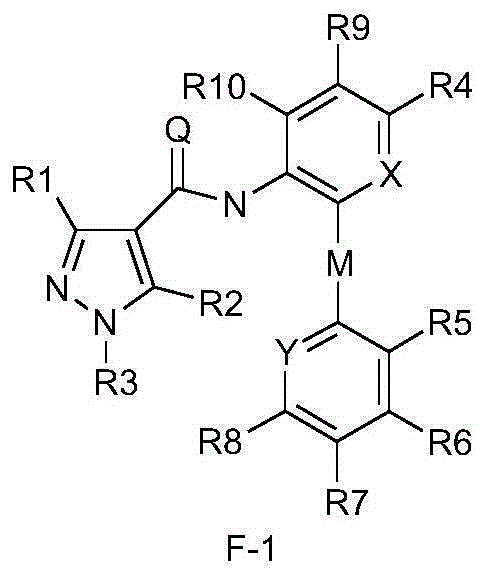
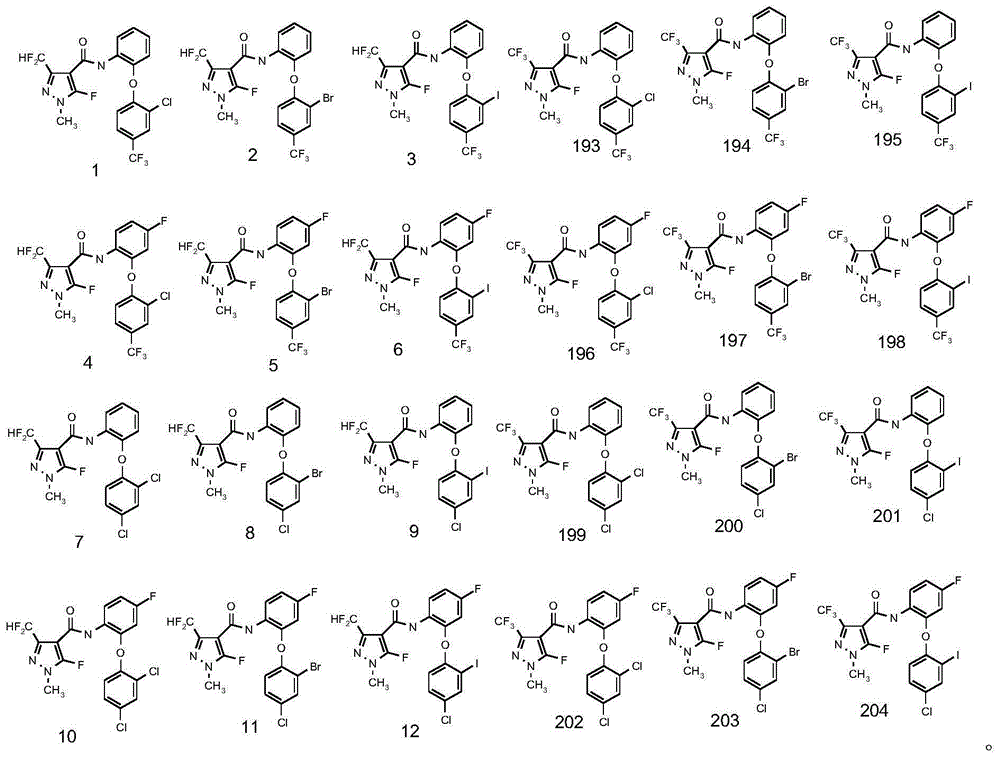
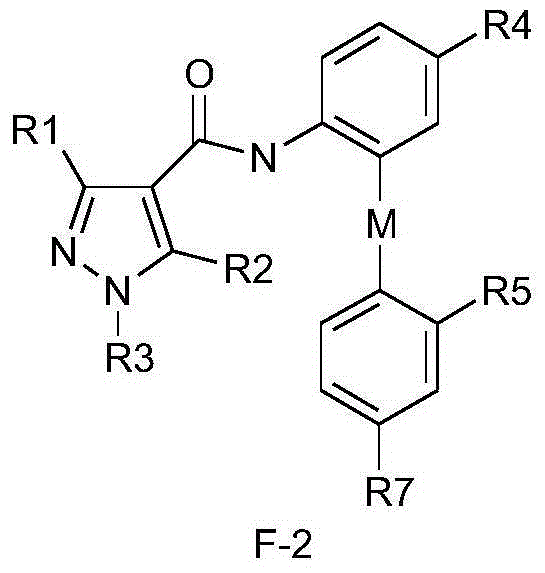
Diphenyl ether structure-containing pyrazolecarboxamide compound and its preparation method and use
A technology of pyrazole amide and diphenyl ether, applied in the field of pyrazole amide compounds, can solve the problems of no control effect and zero control effect on cucumber powdery mildew
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: compound 1 is synthesized
[0084]
[0085] Step (1): Synthesis of 1-methyl-3-difluoromethyl-5-hydroxy-1H-pyrazole
[0086] In a 500ml three-neck flask, add 42 grams of ethyl difluoroacetoacetate in 300ml of toluene, add 10 grams of acetic acid, cool, add 1.1 times the molar amount of 40% methylhydrazine aqueous solution under stirring, and drop it for about 1 hour. , the reaction system was raised to room temperature, and reacted overnight. The next day, 100ml of water was added to the system, the toluene layer was separated, the organic layer was dried with anhydrous magnesium sulfate, and the toluene was distilled off under reduced pressure to obtain 32 grams of a light red solid. The light red solid is 1-methyl-3-difluoromethyl-5-hydroxy-1H-pyrazole, with a melting point of 130-132°C, and it is used in the next reaction without purification.
[0087] Step (2): Synthesis of 1-methyl-3-difluoromethyl-5-chloro-4-formaldehyde-1H-pyrazole
[0088] Add ...
Embodiment 2
[0100] Embodiment 2: compound 2 is synthesized
[0101]
[0102] Step (1): 2-bromo-4-trifluoromethyl-phenol synthesis
[0103] Dissolve 32 g of p-trifluoromethylphenol in 200 ml of dichloromethane, add equimolar liquid bromine dropwise under cooling, and the dropwise addition is completed in about 2 hours, then stir at room temperature for 12 hours, and wash the system with saturated sodium bicarbonate until it becomes neutral. After drying, the solvent was distilled off to obtain 40 g of viscous liquid. The obtained viscous liquid is 2-bromo-4-trifluoromethyl-phenol, and its NMR data is: 1 HNMR (600MHZ, CDCl3 / TMS) δ = 7.75 (1H, s), 7.47 ~ 7.49 (1H, d), 7.08 ~ 7.10 (1H, d), 6.21 (1H, s) ppm, used for the next reaction without purification .
[0104] Step (2): Synthesis of 2-bromo-1-(2-nitro-phenoxy)-4-trifluoromethylbenzene
[0105] Add 14.1 grams (0.1mol) of 2-fluoronitrobenzene into a 150ml single-necked bottle, add 70ml N, N-dimethylformamide, add 1.5 times the molar...
Embodiment 3
[0111] Embodiment 3: compound 4 is synthesized
[0112]
[0113] Step (1): Synthesis of 2-chloro-1-(2-nitro-5-fluoro-phenoxy)-4-trifluoromethylbenzene
[0114] Add 15.9 grams (0.1mol) of 2,4-difluoronitrobenzene into a 150ml single-necked bottle, add 150ml N,N-dimethylformamide, add 1.5 times the molar amount of potassium carbonate, and cool the system to 10°C. Then slowly add 19.6 grams (0.1mol) of 2-chloro-4-trifluoromethylphenol for about 1 hour, keep the temperature and react for 2 hours, then pour the system into 500 ℃ of water, extract with ethyl acetate, and dry with anhydrous magnesium sulfate , evaporated the solvent, and purified by column chromatography to obtain 10 g of the product. The product is 2-chloro-1-(2-nitro-5-fluoro-phenoxy)-4-trifluoromethylbenzene with a melting point of 118-120°C and the NMR data are as follows:
[0115] 1 HNMR (600MHZ, CDCl3 / TMS): δ=8.13~8.16(1H,q), 7.80(1H,s), 7.56~7.58(1H,d), 7.14~7.15(1H,d), 6.99~7.02(1H ,m), 6.62~6.64(1H,q)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com