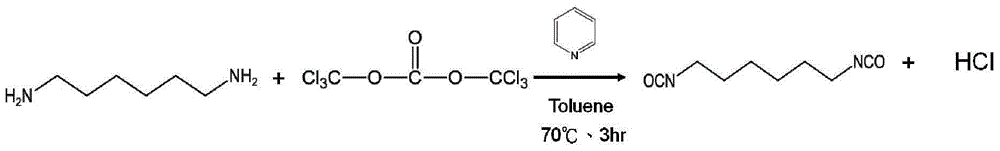
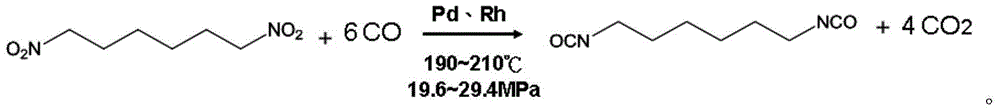
Two-stage method and one-pot synthesis method for preparing aliphatic diisocyanate
A technology of diisocyanate and aliphatic diamine, applied in the field of aliphatic diisocyanate, can solve the problem of many side reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0140] Synthesis of N,N'-Hexamethylenedicarbamate (HMBPC)
[0141] Hexamethylenediamine (HMDA, 6.0 g, 51.7 mmol), diphenyl carbonate (DPC, 22.67 g, 106 mmol) were added to a 500 ml three-necked flask containing 100 ml ethylene glycol diethyl ether. The mixture was stirred at room temperature with a magnetic stir bar. One neck of the bottle was equipped with a thermometer and filled with nitrogen, and the other neck was connected to a condenser filled with water, the upper end of which was connected to an oil seal. The reaction was carried out for 2 hours. The reaction was terminated when IR analysis indicated the absence of 4,4'-diaminodiphenylmethane and monocarbamate intermediates.
[0142] After the reaction was complete, the reaction mixture was continued to be stirred and the temperature was slowly lowered to room temperature. Precipitation of a white crystalline product was observed. Suction filtration is then applied to obtain the product. and dried in a vacuum ove...
example 2
[0148] Synthesis of N,N'-Hexamethylene Dicarbamate Using Different Reaction Solvents
[0149] Using phenol, toluene, methylcyclohexane, 1-bromopropane, ethylene glycol diethyl ether (1,2-diethoxyethane, EGDEE) etc. as the reaction solvent, the same procedure and analysis as in Example 1 were carried out. Synthetic conditions are summarized in Table 1. The results showed that ethylene glycol diethyl ether with low polarity was the best choice. The highest yield of 90.3% methyl hexamethylene dicarbamate (HDU) and the lowest 8.54% by-product urea can be obtained.
[0150] Table 1: The effect of different low polar solvents on the yield of HMBPC synthesis
[0151]
example 3
[0153] Synthesis of Methyl Hexamethylenedicarbamate (HMBPC) Using Different Amounts of Diphenyl Carbonate (DPC)
[0154] The same procedure and analysis as in Example 1 were performed except that the amount of diphenylcarbonate (DPC) was changed as shown in Table 2. As can be seen from Table 2, except when the amount of diphenylcarbonate (DPC) increases to 4 times of MDA molar number, can obtain the hexamethylene dicarbamic acid methyl ester that highest productive rate is 95% (HDU) urea with a minimum of 2.04% by-products.
[0155] Table 2: Effect of different DPC ratios on HMBPC yield in ethylene glycol diethyl ether (EGDEE)
[0156]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com