Synthesis method of terpenoid and glycosylation products thereof in synthesis route of mogrol
A glycosylation and product technology, applied in the biological field, can solve problems such as inaccurate verification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment is carried out under the premise of technical solution of the present invention, has provided detailed embodiment and specific operation process, embodiment will help to understand the present invention, but protection scope of the present invention is not limited to following embodiment . Embodiment 1, the construction of Saccharomyces cerevisiae recombinant strain SY2
[0022] The construction of Saccharomyces cerevisiae recombinant strain SY2 comprises the following steps:
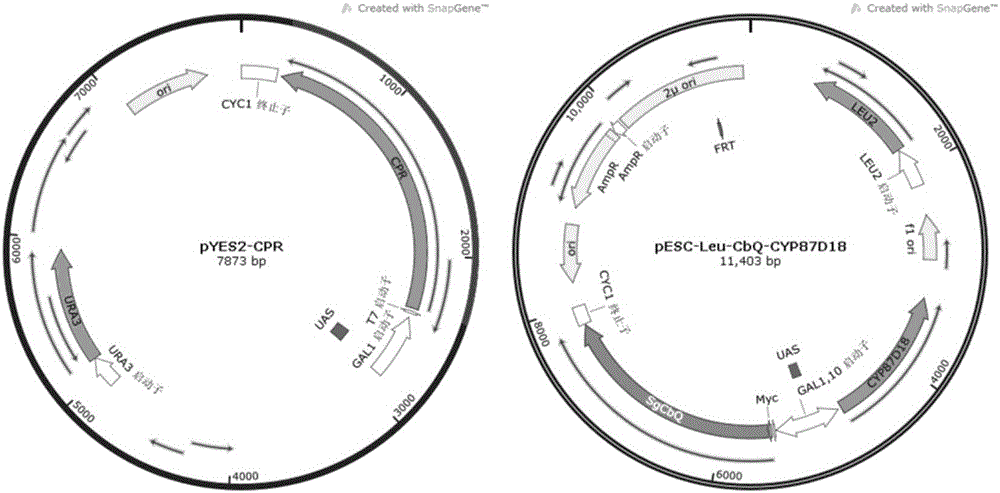
[0023] 1. Construction of Saccharomyces cerevisiae recombinant plasmid pESC-Leu-CbQ
[0024] The progene SgCbQ (GeneBank ID NO: HQ128567) has been confirmed as a cucurbitanedienol synthase. By analyzing the codon usage preference of Saccharomyces cerevisiae, the SgCbQ gene was codon-optimized and handed over to Wuxi Qinglan Biotechnology Co., Ltd. for synthesis. The sequence of the synthesized SgCbQ gene fragment is shown in SEQ ID NO.1; then the synthesized The SgCbQ gene was dige...
Embodiment 2
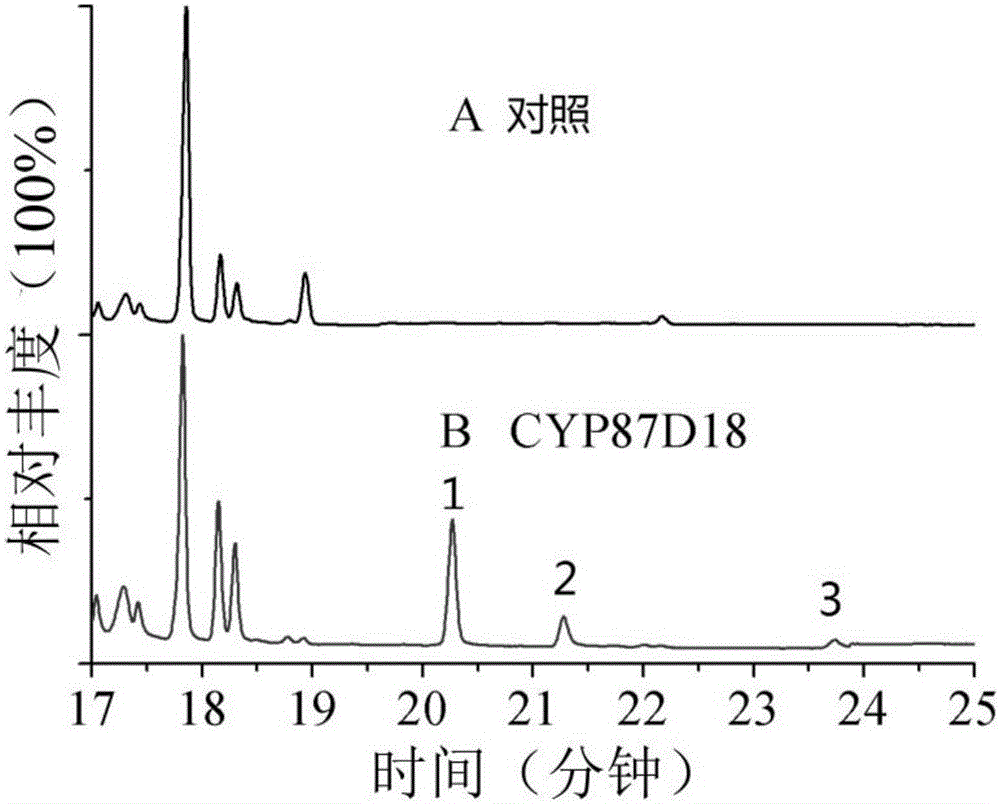
[0032] Example 2, Saccharomyces cerevisiae recombinant strain SY2 synthesized three kinds of cucurbitane-type triterpenoids with glucose as substrate
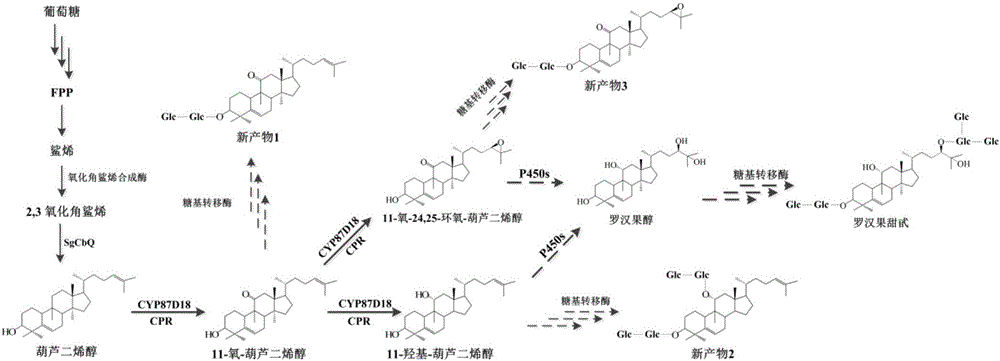
[0033] Recombinant Saccharomyces cerevisiae SY2 uses 2, 3-oxidized squalene contained in its own body as a substrate to obtain cucurbitadienol through cucurbitadienol synthase, which is further catalyzed by cytochrome P450 CYP87D18 to synthesize new products, The identification of new products includes the following processes:
[0034] 1. Fermentation of Saccharomyces cerevisiae recombinant strains SY2 and SY1
[0035] Pick a single colony from a plate containing Saccharomyces cerevisiae recombinant strain SY2 and inoculate it in 20 mL yeast synthetic medium lacking uracil and leucine (supplemented with 20 g / L glucose and 13 mg / L hemin) , cultured at 30°C and 200rpm for 48 h to proliferate yeast cells in large quantities; the cells were collected and washed three times with sterilized deionized water; then transferred to yea...
Embodiment 3
[0052] Example 3. In vitro P450 enzyme CYP87D18 reaction to determine the synthesis sequence of related products in the mogrosanol synthesis pathway
[0053] The synthesis sequence of three products catalyzed by cytochrome P450 CYP87D18 to cucurbitanedienol was determined by microsome preparation. The specific operation process is as follows:
[0054] 1. Induction of cytochrome P450 CYP87D18 in Saccharomyces cerevisiae recombinant strain SY2
[0055] Similar to the cultivation method of the SY2 strain and the induction method of related enzymes in Example 2. In order to compare the in vitro enzyme-catalyzed reaction of cytochrome P450 CYP87D18, the recombinant strain SY1 of Saccharomyces cerevisiae also adopted the cell culture and enzyme induction methods as in Example 2 at the same time.
[0056] 2. Preparation of microsomes
[0057] Centrifuge 50ml of the induced bacterial solution at 5000 rpm for 5 minutes to collect the bacterial cells, and wash twice with distilled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com