Method for detecting clenbuterol hydrochloride residues based on blood glucose meter
A technology of clenbuterol hydrochloride and blood glucose meter, which is applied in the field of detecting clenbuterol hydrochloride residues, can solve the problem of high operator requirements, poor portability and operability of electrochemical workstations, and limited amount of coated antigens and other problems, to achieve the effects of high sensitivity, improved enrichment efficiency and impurity purification efficiency, and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
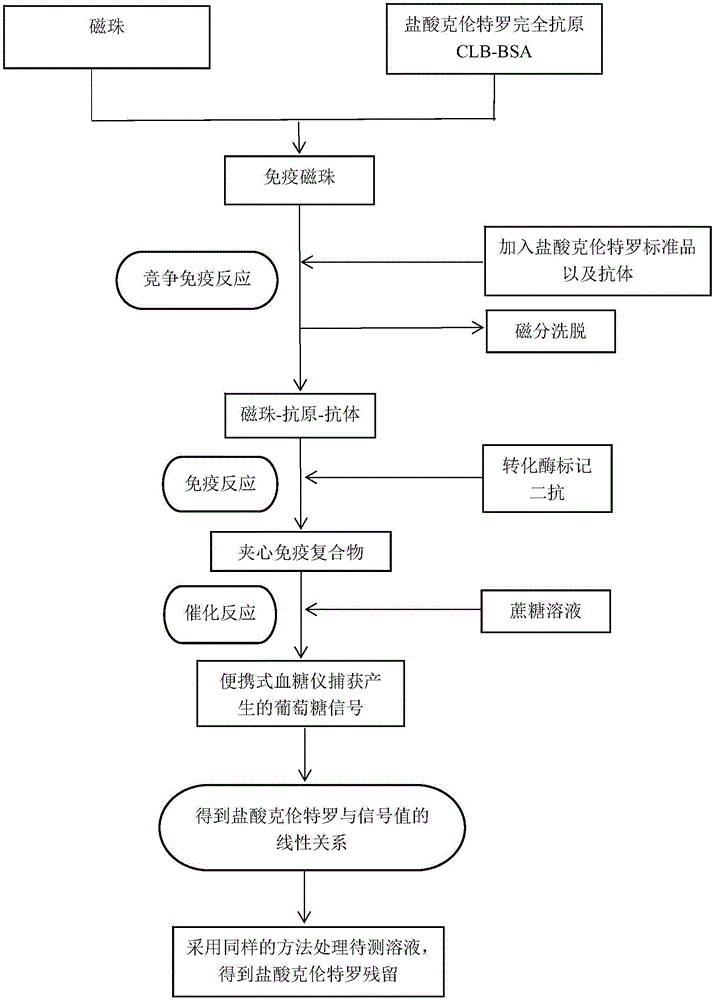
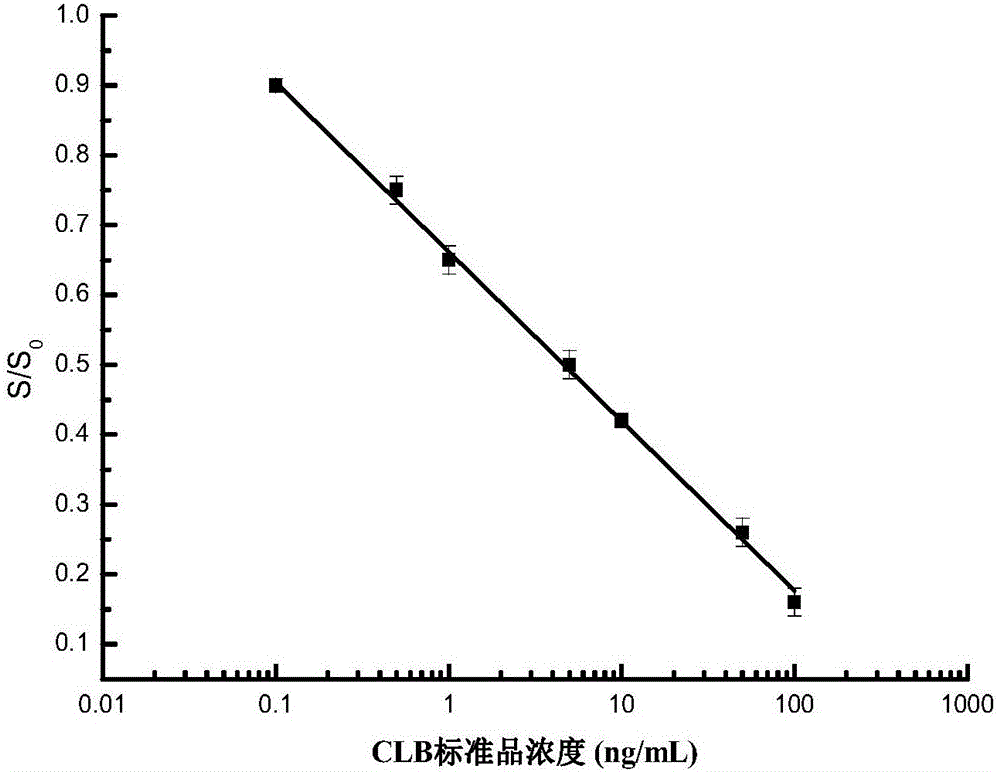
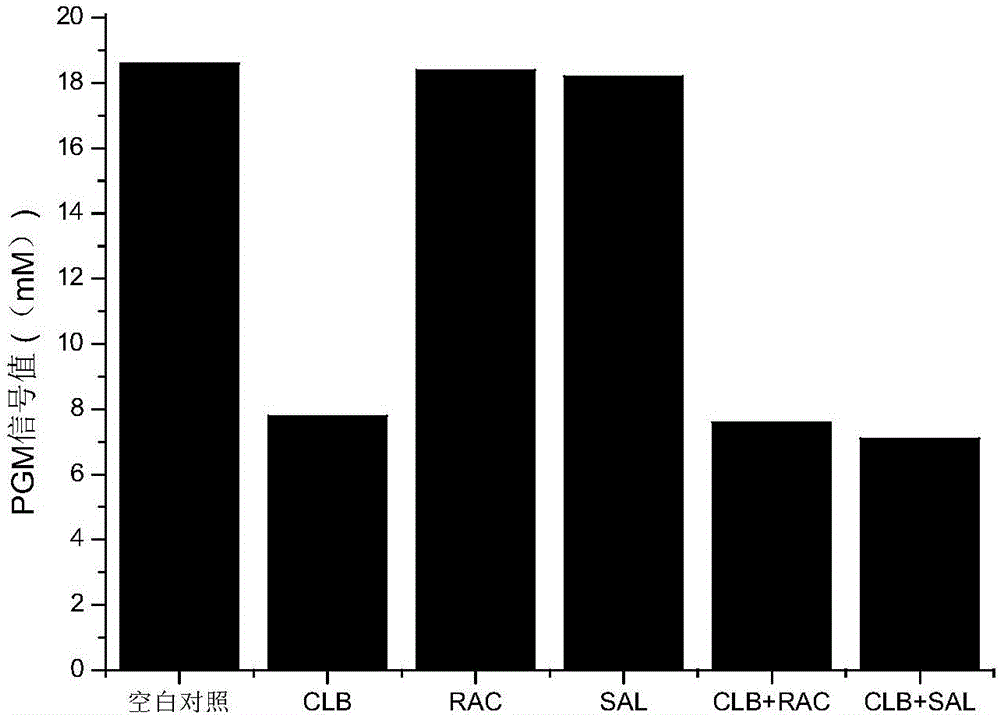
[0028] A method for detecting clenbuterol hydrochloride residues based on a blood glucose meter, the flow chart is shown in figure 1 ,Specific steps are as follows:
[0029] 1) Preparation of immunomagnetic bead probes
[0030] a. Add 200 μL MES solution (15 mM, pH 6.0) containing 5 mg / mL EDC to 2 mg carboxyl magnetic beads, activate at room temperature for 30 min, and magnetically separate the supernatant;
[0031] b. Add 500 μL MES solution containing 0.1 mg CLB-BSA, react overnight at room temperature, and magnetically separate the supernatant;
[0032] c. Add PBS (0.01M, pH 6.0) solution containing 2% OVA, block at 37°C for 2 hours, and magnetically separate the supernatant to obtain immunomagnetic beads;
[0033] d. Add PBS solution containing 0.1% OVA to resuspend the magnetic beads (magnetic bead concentration: 1 mg / mL), and store in a refrigerator at 4°C until use.
[0034] 2) Preparation of colloidal gold / invertase / secondary antibody complex (ie enzyme-labeled seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com