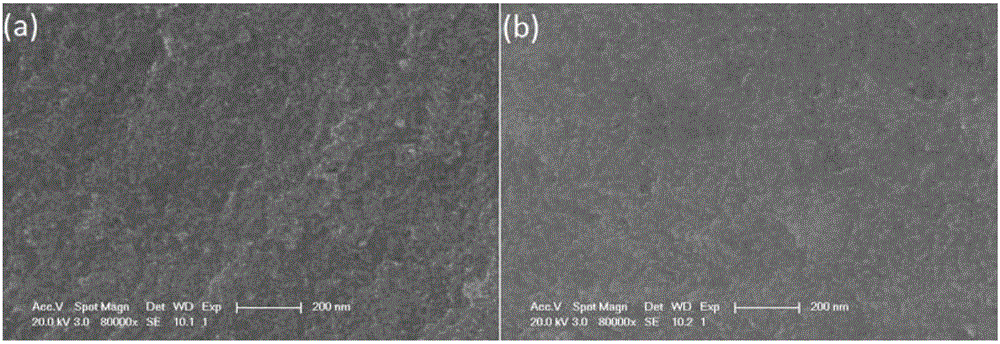
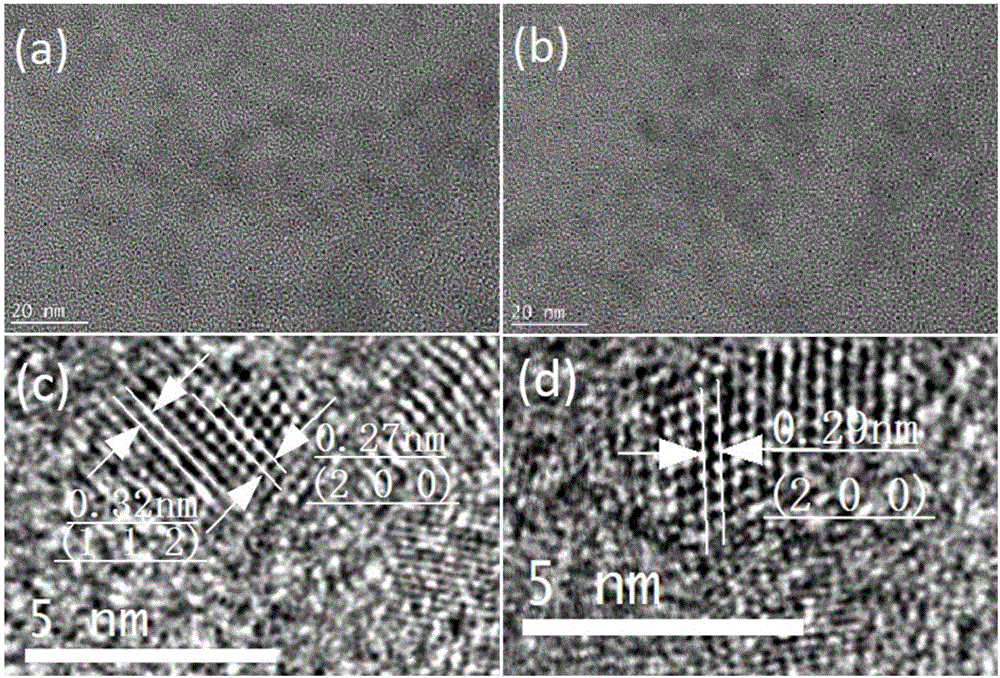
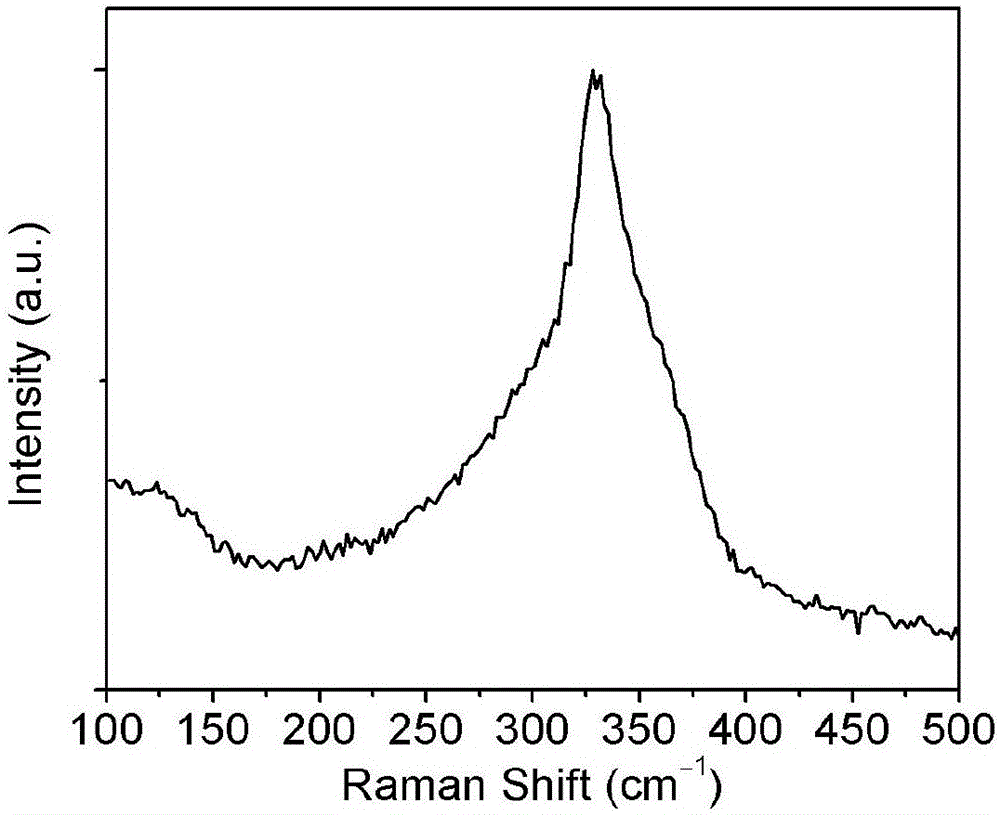
Preparation method of copper zinc tin sulfur selenium nanocrystal
A technology of copper, zinc, tin, sulfur, selenium and nanocrystals, which is applied in the field of nanomaterials, can solve the problems of high price, easy pollution of the environment, and high energy consumption, and achieve the effects of low cost, simple preparation, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment prepares Cu according to the following steps 2 ZnSn(S 1-x Se x ) 4 Nanocrystalline (x=0):
[0046] a, dissolving 10.0mmol S powder into 9.0mL mass concentration is 20.0% (NH 4 ) 2 In the S solution, magnetically stir for 1 hour to completely dissolve; then add 5.0 mmol of Sn powder and continue stirring for 0.5 hour to completely dissolve the Sn powder to obtain a clear yellow-green solution A;
[0047] b. Mix 5.0mmol Zn(NO 3 ) 2 ·6H 2 O was added to 6.0 mL of ammonia solution with a mass concentration of 25.0%, and ultrasonically treated for 3 minutes to obtain a colorless and transparent solution B;
[0048] c, respectively prepare 70mL 1.0M thiourea aqueous solution (colorless and clear) and 3mL 1.68M Cu(NO 3 ) 2 aqueous solution (dark blue), then Cu(NO 3 ) 2 The aqueous solution was added dropwise to the thiourea aqueous solution, and magnetically stirred for 20 minutes to obtain a colorless and clear solution C;
[0049] d. Pour solutio...
Embodiment 2
[0058] This embodiment prepares Cu according to the following steps 2 ZnSn(S 1-x Se x ) 4 Nanocrystalline (x=0.52):
[0059] a, 4mmol S powder and 6mmol Se powder are dissolved to 9.0mL mass concentration and are 20.0% (NH 4 ) 2 In the S solution, magnetically stir for 1 hour to completely dissolve; then add 5.0 mmol of Sn powder and continue stirring for 1 hour to completely dissolve the Sn powder to obtain black solution A;
[0060] b. Mix 5.0mmol Zn(NO 3 ) 2 ·6H 2 O was added to 6.0 mL of ammonia solution with a mass concentration of 25.0%, and ultrasonically treated for 3 minutes to obtain a colorless and transparent solution B;
[0061] c, respectively prepare 70mL 1.0M thiourea aqueous solution (colorless and clear) and 3mL 1.68M Cu(NO 3 ) 2 aqueous solution (dark blue), then Cu(NO 3 ) 2 The aqueous solution was added dropwise to the thiourea aqueous solution, and magnetically stirred for 20 minutes to obtain a colorless and clear solution C;
[0062] d. Pou...
Embodiment 3
[0069] This embodiment prepares Cu according to the following steps 2 ZnSn(S 1-x Se x ) 4 Nanocrystalline (x=0.65):
[0070] a, dissolving 10.0mmol Se powder into 9.0mL mass concentration is 20.0% (NH 4 ) 2 In the S solution, stir magnetically for 1 hour to completely dissolve; then add 5.0 mmol of Sn powder and continue stirring for 0.5 hour to completely dissolve the Sn powder to obtain black solution A;
[0071] b. Add 5.0mmol Zn(NO 3 ) 2 ·6H 2 O was added to 6.0 mL of ammonia solution with a mass concentration of 25.0%, and ultrasonically treated for 3 minutes to obtain a colorless and transparent solution B;
[0072] c, respectively prepare 70mL 1.0M thiourea aqueous solution (colorless and clear) and 3mL 1.68M Cu(NO 3 ) 2 aqueous solution (dark blue), then Cu(NO 3 ) 2 The aqueous solution was added dropwise to the thiourea aqueous solution, and magnetically stirred for 20 minutes to obtain a colorless and clear solution C;
[0073] d. Pour solution A and sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com