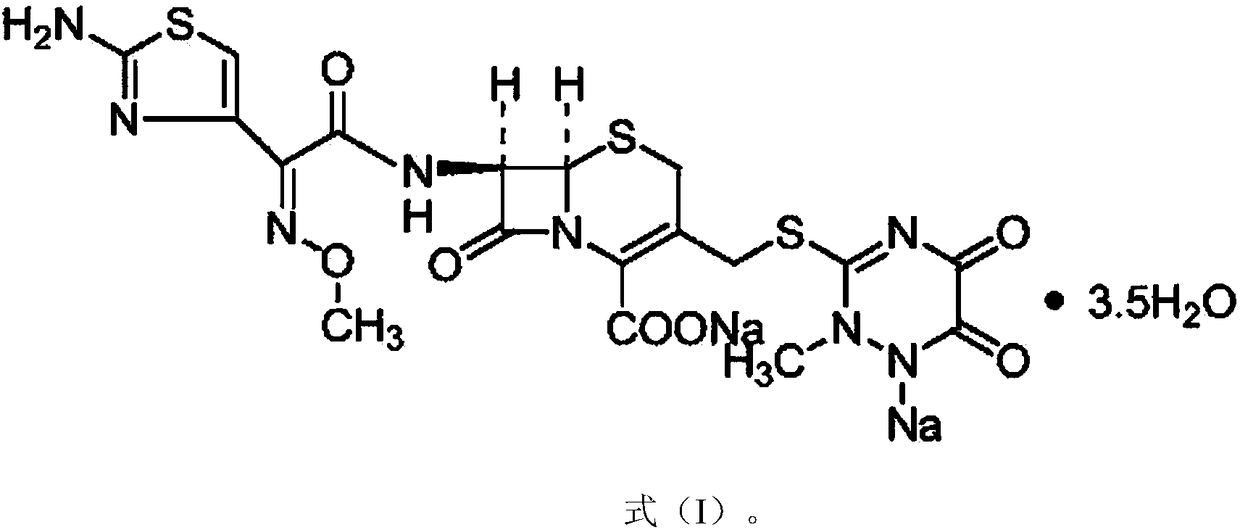
A kind of anti-infection drug ceftriaxone sodium crystal compound and preparation method thereof
A technology for ceftriaxone sodium and a crystal compound, which is applied in the field of medicine and achieves the effects of good clarity, reduced incidence of adverse reactions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
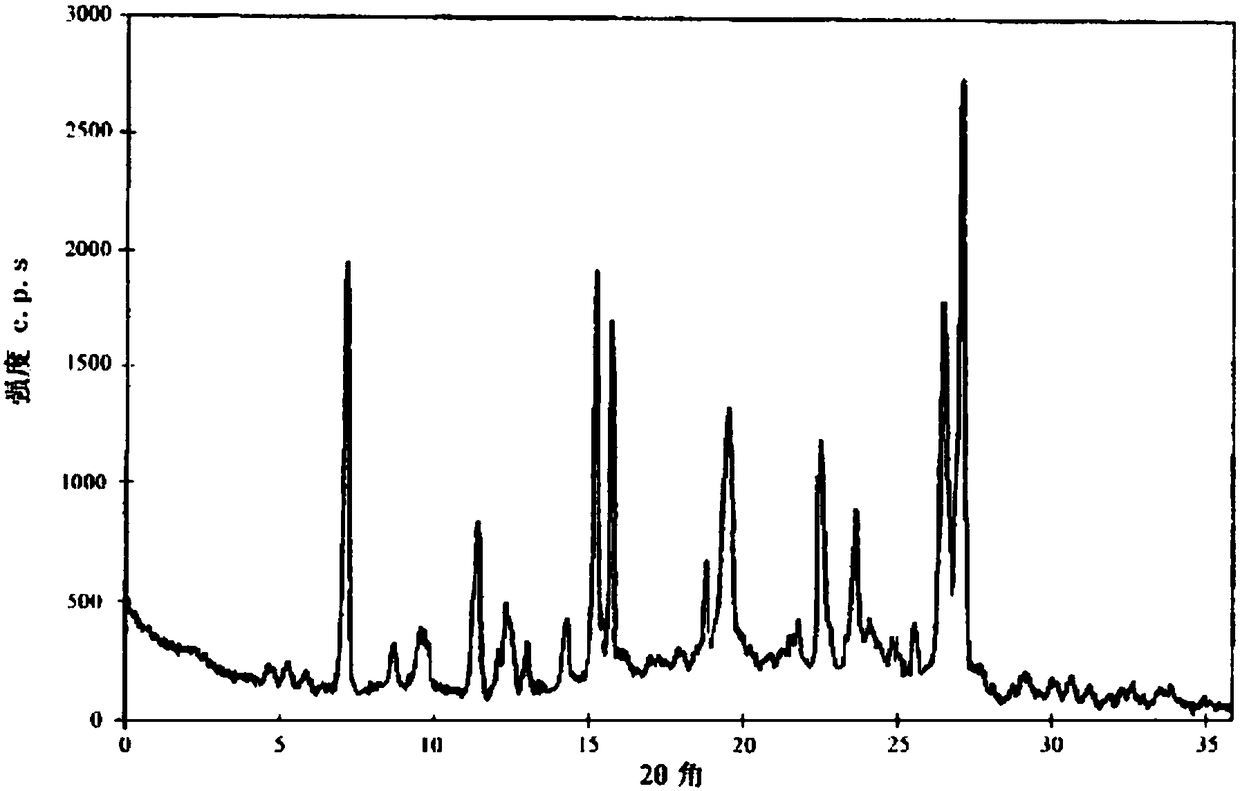
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 ceftriaxone sodium crystal compound
[0044] (1) under the sound field that frequency is 30KHz, output power is 50W, with the stirring velocity of 200 rev / mins, ceftriaxone sodium crude product is added into the ethanol that volume is 6 times of ceftriaxone sodium weight, water while stirring. 1. In the mixed solution of ethyl acetate, the volume ratio of ethanol, water, and ethyl acetate is 2.5:4.5:1, the temperature is raised to 35-45°C, and stirred until dissolved;
[0045] (2) With a stirring speed of 200 revs / min, while stirring, adding volume is the mixed solution of ethyl acetate, hexanaphthene which is 3 times of ceftriaxone sodium weight, and the volume ratio of ethyl acetate and hexanaphthene is 3.5: 2.5;
[0046] (3) After adding the mixed solution of ethyl acetate and cyclohexane, under a sound field with a frequency of 20KHz and an output power of 15W, cool down to 0-2°C at 3°C / hour, grow crystals for 3-6 hours, and wash , ...
Embodiment 2
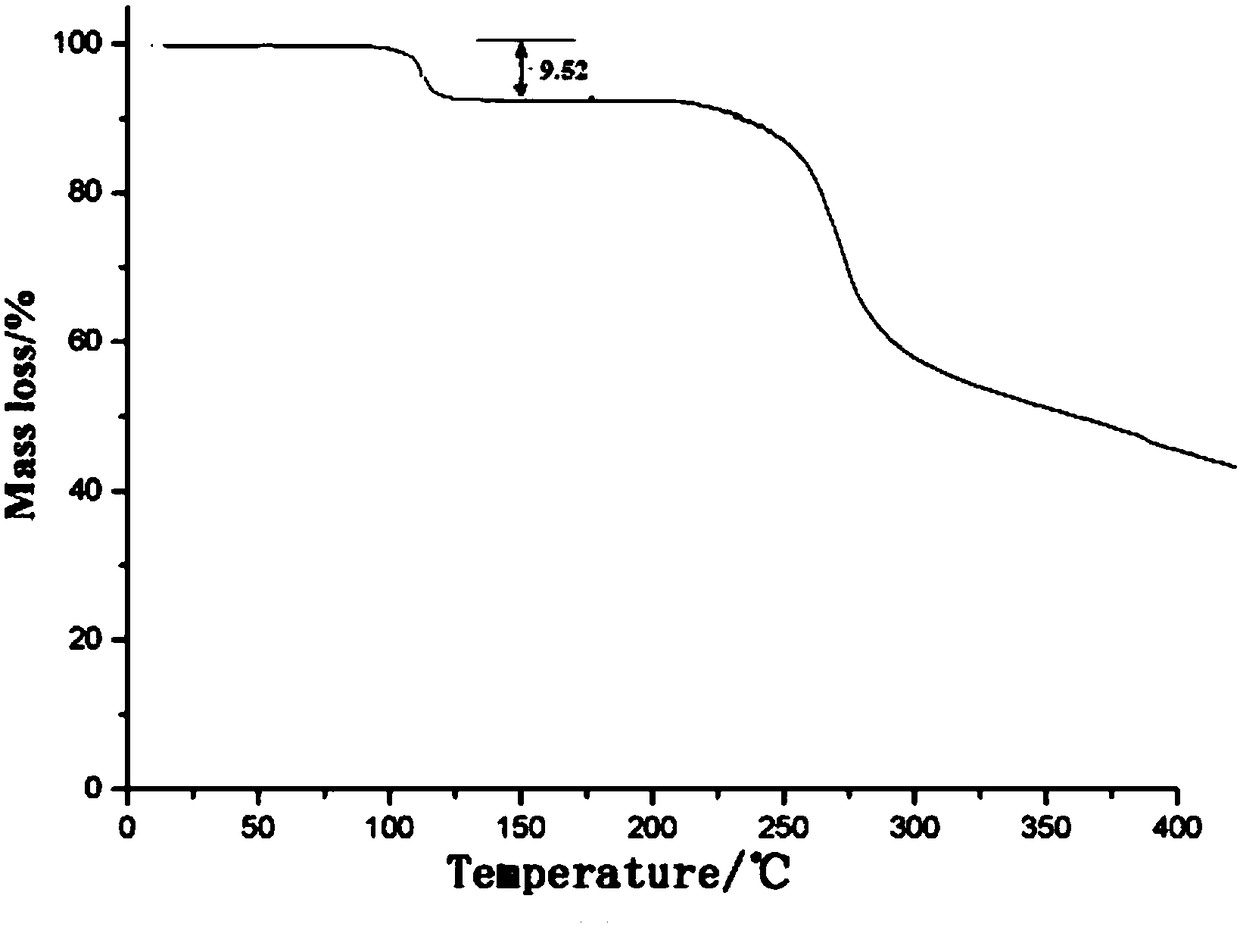
[0053] Determined by thermogravimetric analysis, the results are as follows figure 2 As shown, the crystal water content is 9.53wt%, which is basically consistent with the theoretical value. The preparation of embodiment 2 ceftriaxone sodium crystalline compound
[0054] (1) under the sound field that frequency is 25KHz, output power is 60W, with the stirring velocity of 260 rev / mins, ceftriaxone sodium crude product is added into the ethanol that volume is 5 times of ceftriaxone sodium weight, water while stirring. 1. In the mixed solution of ethyl acetate, the volume ratio of ethanol, water, and ethyl acetate is 2.5:4.5:1, the temperature is raised to 35-45°C, and stirred until dissolved;
[0055] (2) With a stirring speed of 260 revs / min, while stirring, add volume as the mixed solution of ethyl acetate, cyclohexane that ceftriaxone sodium weight 2 times, the volume ratio of ethyl acetate and cyclohexane is 3.5: 2.5;
[0056] (3) After adding the mixed solution of ethyl...
Embodiment 3
[0058] The preparation of embodiment 3 ceftriaxone sodium crystalline compound
[0059] (1) under the sound field that frequency is 25KHz, output power is 40W, with the stirring speed of 150 rev / mins, ceftriaxone sodium crude product is added into the ethanol that volume is 7 times of ceftriaxone sodium weight, water while stirring. 1. In the mixed solution of ethyl acetate, the volume ratio of ethanol, water, and ethyl acetate is 2.5:4.5:1, the temperature is raised to 35-45°C, and stirred until dissolved;
[0060] (2) With a stirring speed of 150 revs / min, while stirring, adding volume is the mixed solution of ethyl acetate, hexanaphthene which is 4 times of ceftriaxone sodium weight, and the volume ratio of ethyl acetate and hexanaphthene is 3.5: 2.5;
[0061] (3) After adding the mixed solution of ethyl acetate and cyclohexane, under a sound field with a frequency of 15KHz and an output power of 10W, cool down to 0-2°C at 4°C / hour, grow crystals for 3-6 hours, and wash ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com