A force- and light-responsive self-assembly based on dual fluorescent chromophores and its preparation method
A dual-response, self-assembly technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as difficult aggregates, lack of assembly driving force, and lack of attention, avoiding complex processes and achieving excellent crystallization. performance, easy to prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
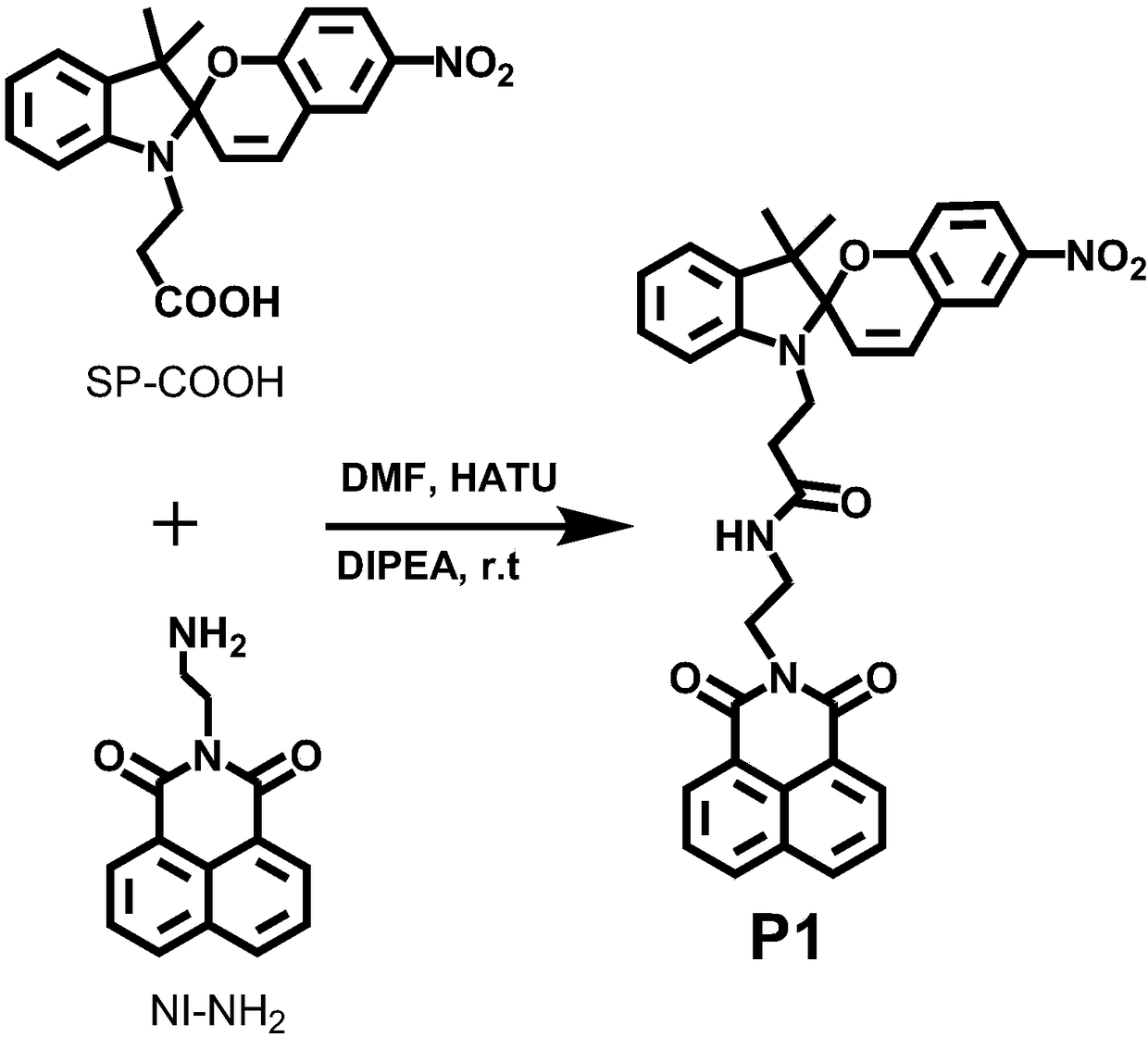
[0036] Spiropyran (SP-COOH) (300mg, 0.789mmol, structural formula as shown above) with a single carboxyl group and the condensing agent 2-(7-azobenzotriazole)-N,N,N' , N'-tetramethyluronium hexafluorophosphate (HATU) (600mg, 1.578mmol) was added to two reaction tubes, and then the basic reagent N,N-diisopropylethylamine (DIEA) (1.32mL, 8mmol ), then add 10mL of anhydrous N,N-dimethylformamide (DMF) until all the reactants are dissolved, stir evenly under nitrogen atmosphere and react at room temperature for 30min; then add naphthalimide (NI -NH 2 ) (227mg, 0.9mmol, the structural formula is as shown above), and the stirring reaction was continued at room temperature for 12h. After the reaction was complete, the excess solvent was removed by rotary evaporation, and the obtained solid crude product was dissolved with methanol (0.5-1 mL), and then precipitated with diethyl ether (30-50 mL). After centrifugation, the precipitate was collected, and the product was pur...
Embodiment 2
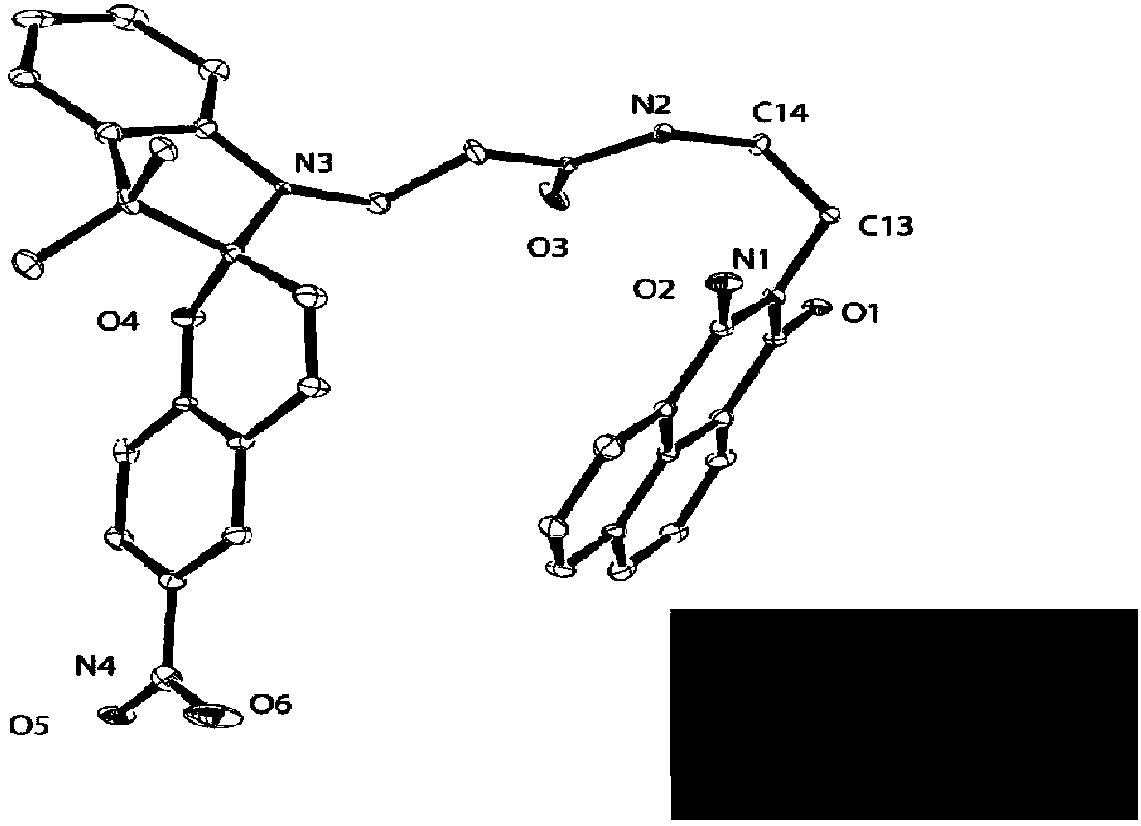
[0040] Take 10 mg (0.02 mmol) of P1 synthesized in Example 1 and dissolve it in 1 mL of dichloromethane, place it in a 10 mL vial, then add 1 mL of a mixed solvent of dichloromethane and n-hexane (v / v=1:1 ), and then add 6 mL of n-hexane. Single crystals can be grown after sealed storage for about 10 hours. The ORTEP diagram of the single crystal analysis and the fluorescence (under 365nm ultraviolet lamp irradiation) photos of the single crystal are as follows figure 2 shown.
Embodiment 3
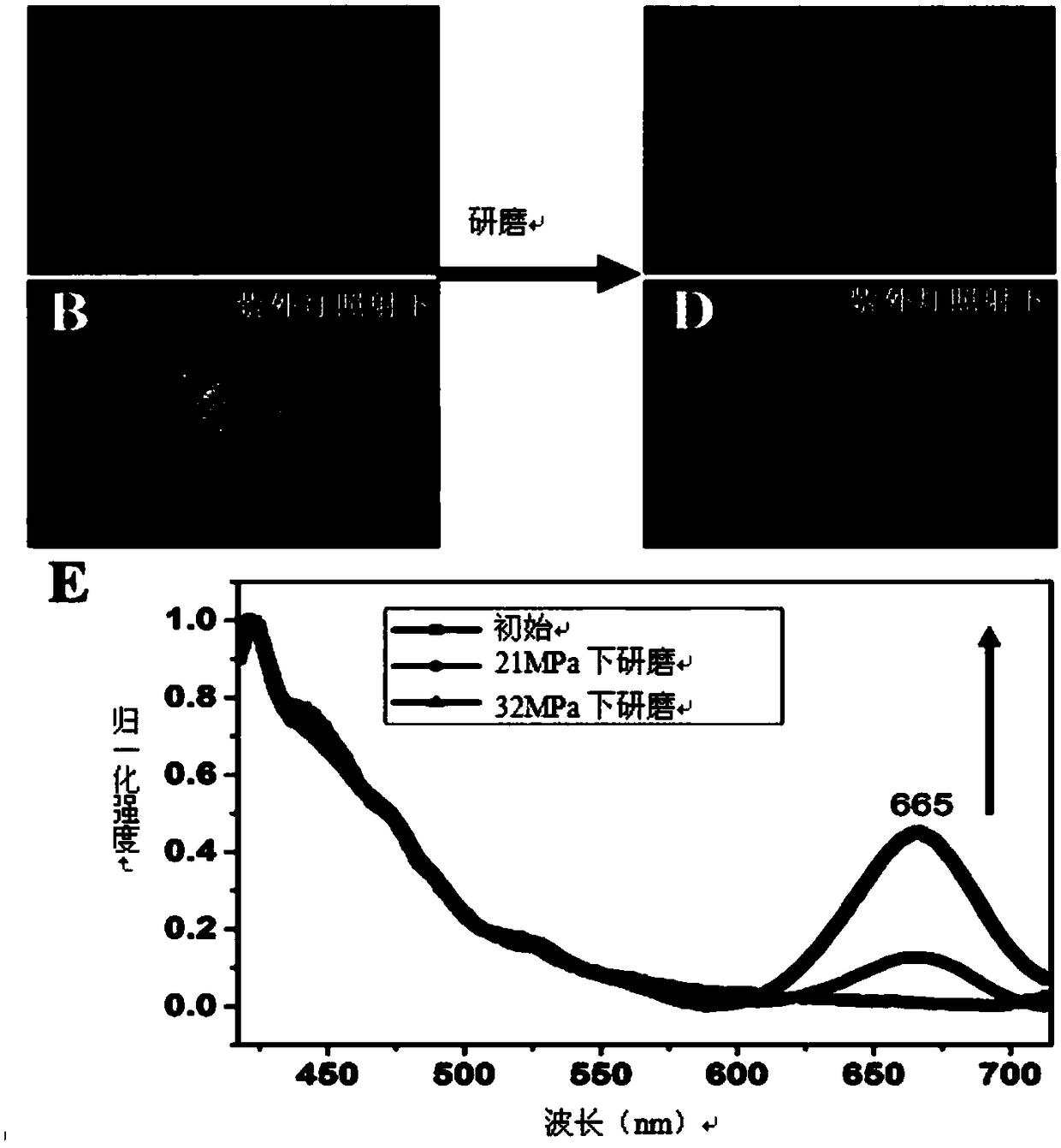
[0042] Mechanofluorescent discoloration effect test: about 10mg (0.02mmol) of P1 synthesized in Example 1 was dissolved in 1.5mL of dichloromethane, added dropwise on the quartz plate, and the solvent was evaporated to dryness in a vacuum oven at 30 degrees Celsius. A mechanofluorescent color-changing film can be obtained, and the film initially has a fluorescent emission peak at 420nm. After erasing and writing with a mechanical force of 21Mpa, in addition to the fluorescence emission peak at 420nm, a new fluorescence emission peak appeared at 665nm. As the mechanical force increases to 32Mpa, the fluorescence emission peak intensity at 665nm also increases. After heat treatment, the new fluorescence emission peak at 665nm gradually weakens, and it can completely return to the original state after heating for 1 hour, and this process can be repeated many times. In order to represent this phenomenon more intuitively, we took pictures. We found that the initial P1 solid-state...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com