Aromatic diboron ester convenient synthesis method suitable for large-scale production
A technology for the synthesis of aromatic diboronic esters and methods, which is applied in the field of convenient synthesis of aromatic diboronic esters, can solve the problems of complicated operation, long purification time, labor consumption, etc., and achieve improved purification efficiency, simple synthetic methods, and reduced labor Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
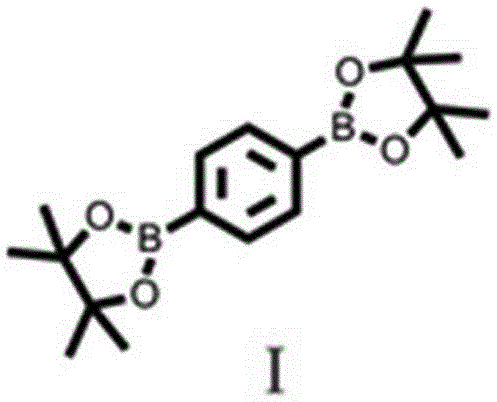
[0019] Synthesis of 1,4-benzenediboronic acid pinacol diester (compound Ⅰ, chemical structure see figure 1 )
[0020] Its synthesis method is as follows:
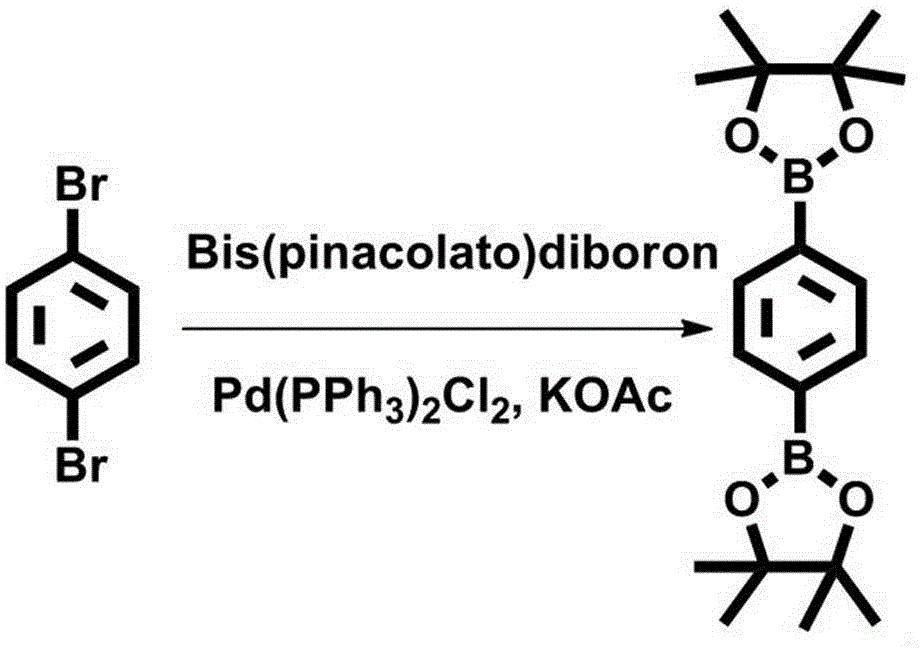
[0021] Measure 2,4 dibromobenzene (500mg, 1.0eq), bis(pinacolate) diboron (1.2g, 2.0eq), PdCl 2 (Ph 3 ) 2 (100mg, 6.2%), KOAc (1.3g, 6.3eq), add them together into a 50ml three-neck flask, replace with nitrogen for 3 times, add 10ml of freshly distilled dioxane, reflux reaction (temperature 90~100℃) After 24h, stop reaction, obtain organic product;
[0022] Spin to dry the solvent, then add 60ml of ethyl acetate, ultrasonically dissolve the organic product, filter through diatomaceous earth, wash the diatomite with 10ml of ethyl acetate, then spin dry the ethyl acetate, add 10ml of petroleum ether, heat it with a hair dryer to promote dissolution, set overnight in the refrigerator; the next day, the solid was filtered by suction and dried in vacuo to obtain 660 mg of the product, yield: 93.6%. 1 H NMR (400 MHz, CDCl ...
Embodiment 2
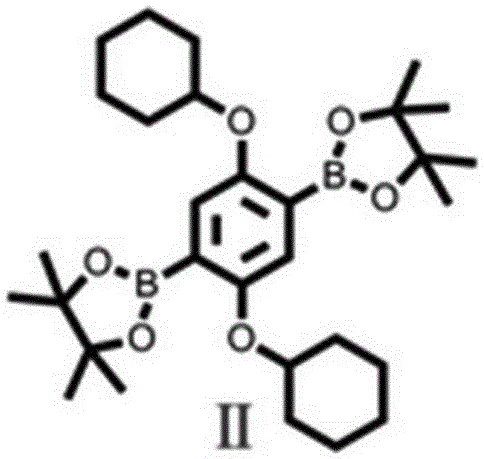
[0025] Synthesis of 1,4 diboronic acid pinacol diester-2,5-tetrahydropyranylbenzene (compound Ⅱ, chemical structure see image 3 ).
[0026] Its synthesis method is as follows:
[0027] 1) Synthesis of 1,4-dibromo-2,5-dimethoxybenzene:
[0028] Add 20.0g (0.145mol, 1.0eq) of hydroquinone dimethyl ether and 55ml of glacial acetic acid into a 250ml reaction bottle, dissolve with ultrasonic; add 15ml of Br 2 (0.290mol, 2.0eq) and 15ml of glacial acetic acid solution, drop it after 1.5 hours, stir at room temperature for 2.5 hours; put it in the upper layer of the refrigerator to cool down to below 10°C (do not let the acetic acid solidify) and filter it; and wash the filter cake with petroleum ether , a white solid was obtained, which weighed 35.6 g after vacuum drying, and the yield was 83.6%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.11 (s, 2H), 3.85 (s, 6H).
[0029] 2) Synthesis of 1,4-dibromohydroquinone:
[0030] In a 100ml single-necked bottle, add 7.1g (24.2mmol, 1.0eq) of 1,4...
Embodiment 3
[0037] Synthesis of 2,7 diboronic acid pinacol diester carbazole (compound Ⅲ, chemical structure see Figure 5 ).
[0038] Its synthesis method is as follows:
[0039] 500mg (1.55mmol, 1.0eq) of 2,7-dibromocarbazole, 980mg (3.86mmol, 2.5eq) of bis(pinacolate) diboron, PdCl 2 (PPh 3 ) 2 Add 100mg (0.142mmol, 0.09eq) and 1.0g (10.2mmol, 6.6eq) of KOAc into a 100ml three-neck flask, replace nitrogen three times, add 50ml of freshly distilled toluene, react at 85°C for 36h, then cool down to terminate the reaction. The reaction solution was filtered through celite, and the celite was washed with ethyl acetate, the ethyl acetate was spin-dried, and recrystallized with petroleum ether or n-hexane (for column chromatography, petroleum ether / ethyl acetate=10 / 1 eluent) to obtain 500 mg of a colorless product with a yield of 76.9%. H NMR (400 MHz, CDCl 3 ) δ 8.10 (d, J = 7.8 Hz, 2H), 8.04 (s, 1H), 7.93 (s, 2H), 7.68(d, J = 7.8 Hz, 2H), 1.38(s, 24H).
[0040] For the specific s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com