Tofacitinib-citrate tablet and method for preparing tofacitinib-citrate tablet
A technology of citric acid and cloth tablets, which is applied in anti-inflammatory agents, pill delivery, and pharmaceutical formulations. The effect of punching and process reproducibility is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
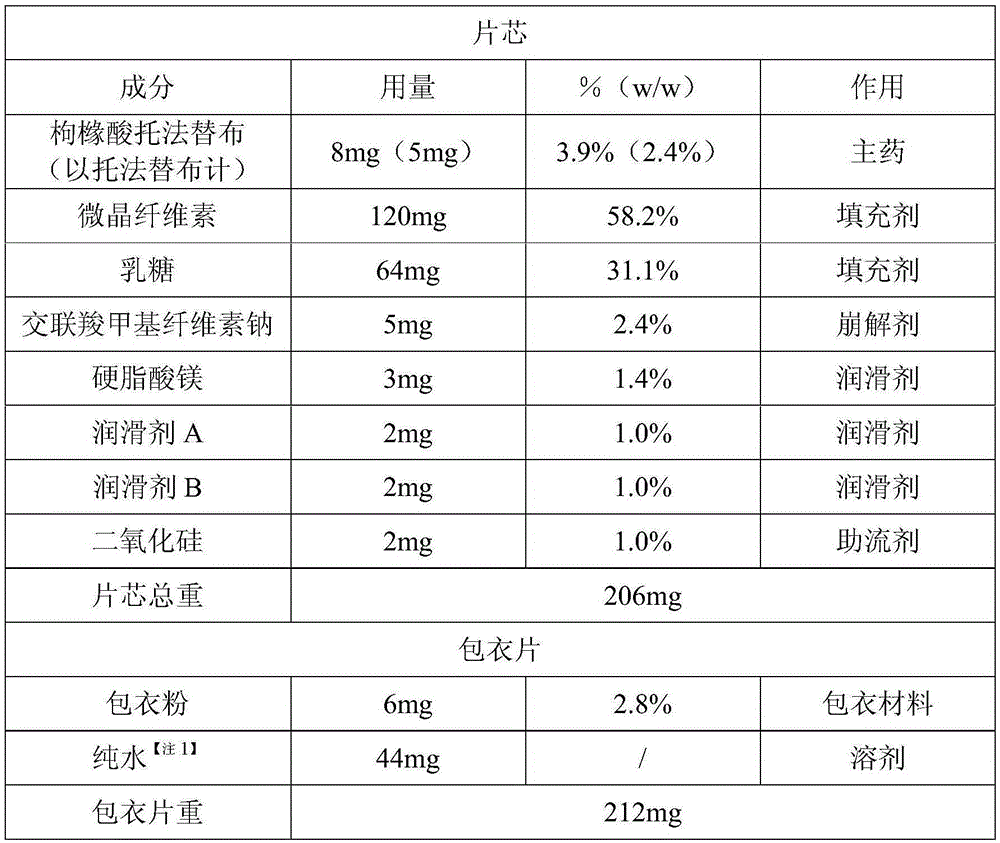
[0040] (1) Formula composition:
[0041] Table 1-1 Recipe 1
[0042]
[0043]
[0044] (2) Preparation:
[0045] ①Pretreatment: Pass magnesium stearate through a 40-mesh sieve, and other raw and auxiliary materials through a 80-mesh sieve, and set aside.
[0046] ②Initial mixing: weigh tofacitinib citrate, microcrystalline cellulose, lactose, and croscarmellose sodium and mix for 20 minutes. Take 3 samples from different positions, detect the content of each point and calculate the RSD, if RSD≤2.0%, it is judged that the mixture is uniform.
[0047] ③Total blending: Weigh the formula amount of magnesium stearate and add it to the above powder, mix for 10 minutes, take samples at the 5th and 10th minutes respectively, take 3 samples at three different positions each time, measure the content of each point and calculate the RSD , RSD≤2.0% judges that the mixture is uniform.
[0048] ④Detect the angle of repose of the powder.
[0049] ⑤ Tablets.
[0050] (3) Testing: ...
Embodiment 2
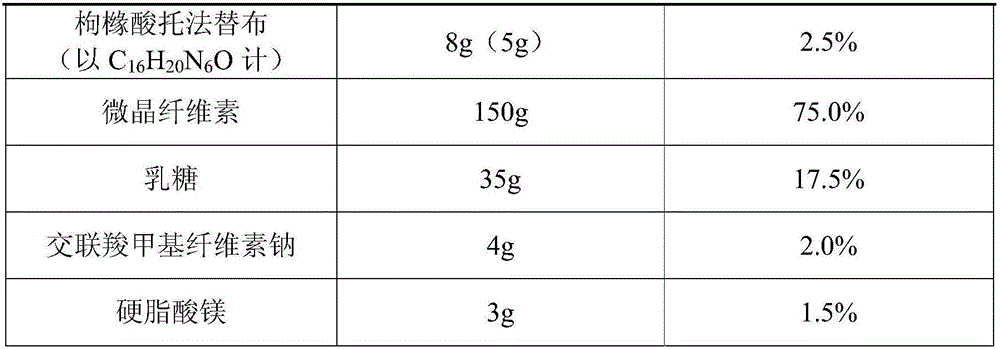
[0056] (1) Formula composition:
[0057] Table 2-1 Recipe 2
[0058]
[0059] (2) Preparation:
[0060] The preparation process is the same as that in Example 1.
[0061] (3) Detection: See Table 2-2 for the results.
[0062] Table 2-2 Determination results of formula 2
[0063]
[0064] (4) Dissolution test; the results are shown in Table 2-3.
[0065] Table 2-3 Dissolution comparison between formulation 2 tablet and reference drug (medium: 0.1mol / L hydrochloric acid)
[0066] sampling time commercially available Recipe 3 5min 87.9% 64.8% 10min 96.8% 88.5% 15min 99.8% 100.4% 30min 102.3% 102.0% 45min 102.5% 101.8%
[0067] (5 Conclusion:
[0068]The disintegration time limit of the formulation 2 tablet was basically the same as that of the control drug, but the initial dissolution rate was significantly slower than that of the control drug. Lactose in the formula is a soluble excipient that has a solubilizing ef...
Embodiment 3
[0070] (1) Formula composition:
[0071] Table 3-1 Recipe 3
[0072]
[0073] (2) Preparation:
[0074] The preparation process is the same as that in Example 1.
[0075] (3) Testing, the results are shown in Table 3-2.
[0076] Table 3-2 Determination results of formula 3
[0077]
[0078] (4) Dissolution determination:
[0079] Table 3-3 Dissolution comparison between formula 3 tablet and reference drug (medium: 0.1mol / L hydrochloric acid)
[0080] sampling time commercially available Recipe 4 5min 87.9% 71.8% 10min 96.8% 89.3% 15min 99.8% 99.4% 30min 102.3% 100.0% 45min 102.5% 102.1%
[0081] (5 Conclusion:
[0082] The initial dissolution rate of the formula 3 tablet was faster than that of the formula 2 tablet, but still slower than that of the reference drug. Therefore, continue to increase the amount of lactose in the formula, accelerate the initial dissolution rate, and design formula 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com