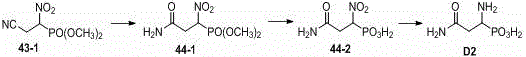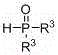Beta-cyano phosphoryl derivatives as well as preparation method and application thereof
A technology of cyanophosphono and derivatives, which is applied in the field of preparation of organic compounds, can solve the problems of harsh reaction conditions, difficult to obtain raw materials, and many reaction steps, and achieve the effects of short reaction time, overcoming toxicity, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment one: the synthesis of 2-phenyl-3-diphenoxyphosphinylpropionitrile
[0039] Using styrene and diphenylphosphine oxide as raw materials, the reaction steps are as follows:
[0040] Add styrene (0.042 g, 0.4 mmol), diphenoxyphosphine (0.081 g, 0.4 mmol), trimethylcyanosilane (0.040 g, 0.4 mmol), CuCl (0.04 g, 0.04 mmol) into the reaction flask, Manganese acetate (0.322 g, 1.2 mmol) and toluene (3 mL) were reacted at 100°C under the protection of argon;
[0041] TLC tracking reaction until complete completion;
[0042] The crude product obtained after the reaction was separated by column chromatography (ethyl acetate: petroleum ether = 1:1) to obtain the target product (yield 73%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 7.92 – 7.81 (m, 2H),7.77 – 7.69 (m, 2H), 7.58 – 7.48 (m, 4H), 7.46 – 7.37 (m, 4H), 7.33 – 7.23(m, 3H), 4.39 (td, J = 9.4, 5.8 Hz, 1H), 3.12 – 2.94 (m, 1H), 2.95 – 2.56 (m, 1H).
Embodiment 2
[0043] Example 2: Synthesis of 2-(4-tolyl)-3-diphenoxyphosphinylpropionitrile
[0044] With 4-methylstyrene, diphenylphosphine oxide as raw material, its reaction steps are as follows:
[0045] Add 4-methylstyrene (0.047 g, 0.4 mmol), diphenoxyphosphine (0.081 g, 0.4 mmol), trimethylcyanosilane (0.040 g, 0.4 mmol), CuCl (0.04 g, 0.04 mmol), manganese acetate (0.322 g, 1.2 mmol) and toluene (3 mL), under the protection of argon, react at 90°C;
[0046] TLC tracking reaction until complete completion;
[0047] The crude product obtained after the reaction was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain the target product (yield 72%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 7.79 (dd, J = 10.7,7.8 Hz, 2H), 7.62 – 7.48 (m, 5H), 7.45 (t, J = 7.3 Hz, 1H), 7.35 (t, J = 6.6Hz, 2H), 7.18 (d, J = 7.8 Hz, 2H), 7.02 (d, J = 7.6 Hz, 2H), 4.41 (d, J = 6.6Hz, 1H), 3.01 (dt, J = 15.0, 7.6 Hz...
Embodiment 3
[0048] Example 3: Synthesis of 2-(4-methoxyphenyl)-3-diphenoxyphosphinylpropionitrile
[0049] With 4-methoxystyrene and diphenylphosphine oxide as raw materials, the reaction steps are as follows:
[0050] Add 4-methoxystyrene (0.054 g, 0.4 mmol), diphenoxyphosphine (0.162 g, 0.8 mmol), trimethylcyanosilane (0.040 g, 0.4 mmol), CuCl (0.04 g , 0.04 mmol), manganese acetate (0.322 g, 1.2 mmol) and N,N-dimethylformamide (3 mL), react at 80°C under argon protection;
[0051] TLC tracking reaction until complete completion;
[0052] The crude product obtained after the reaction was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain the target product (yield 70%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 7.78 (s, 2H), 7.63 –7.49 (m, 5H), 7.43 (s, 1H), 7.35 (s, 2H), 7.21 (s, 2H), 6.72 (s, 2H), 4.42(s, 1H) , 3.73 (s, 3H), 3.03 (s, 1H), 2.80 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com