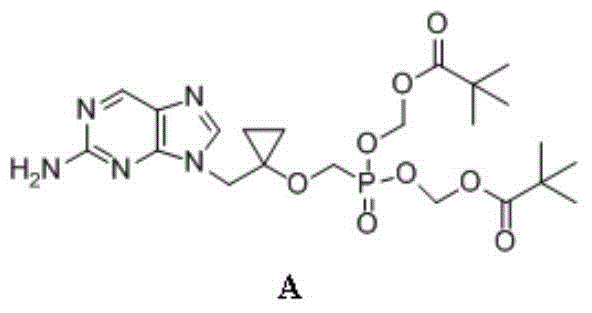
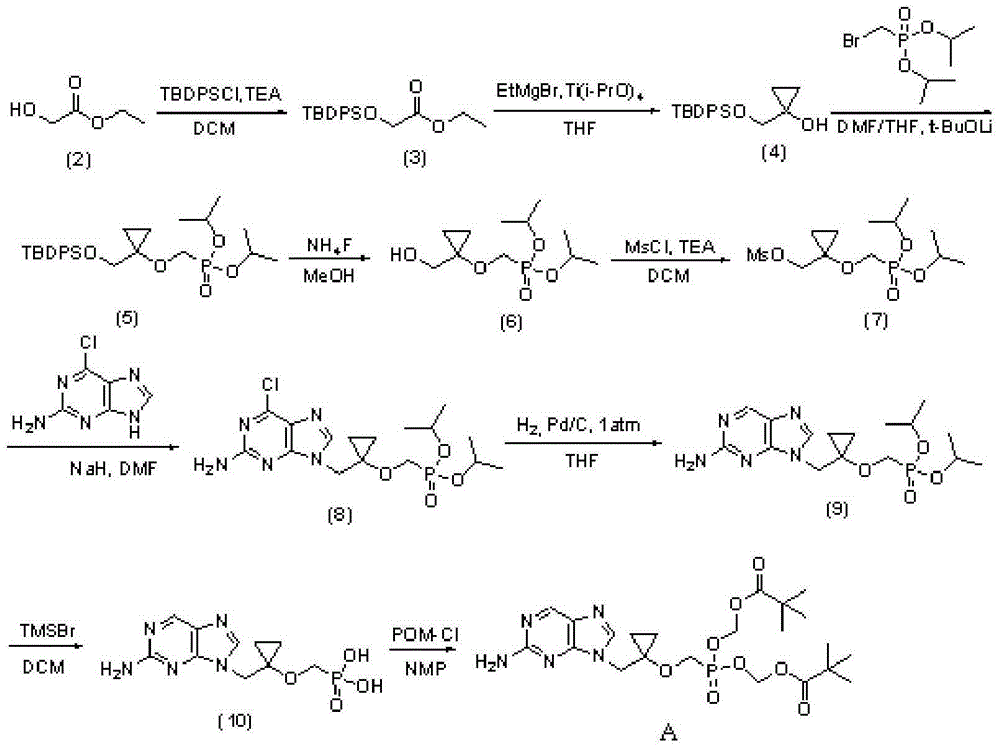
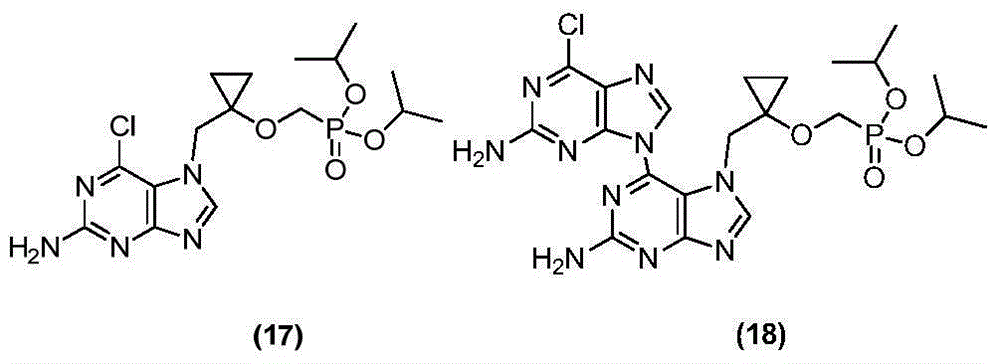
Intermediate compound of medicine LB80380 and preparing method and application thereof
A technology of LB80380 and compound, which is applied to the intermediate compound of LB80380 medicine and the fields of its preparation and use, and can solve problems such as potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Embodiment 1: the preparation of intermediate compound I-1
[0190] step 1. Preparation of ethyl 2-(dibenzylamino)acetate (IV-1)
[0191]
[0192] Dibenzylamine (VI-1) (4.7g, 23.9mmol) was dissolved in dichloromethane (10ml), and ethyl bromoacetate (V-1) (2.0g, 12mmol) was added under stirring at 25°C, and the addition was completed , a white solid precipitated out. Stirring was continued for about 16h, TLC monitored the completion of the reaction, filtered, the filter cake was washed with dichloromethane, the filtrate was concentrated under reduced pressure to obtain a crude solid, which was recrystallized from petroleum ether / ethyl acetate (100:1) to obtain 3.3 g of a white solid, yield was 97.3%.
[0193] HNMR (400MHz, CDCl 3 )δ7.26-7.42 (m, 10H), 4.17 (q, 2H), 3.83 (s, 4H), 3.30 (s, 2H), 1.28 (t, 3H).
[0194] Step 2. Preparation of 1-(dibenzylamino)methylcyclopropanol (II-1)
[0195]
[0196] Under nitrogen protection, ethyl 2-(dibenzylamino)acetate ...
Embodiment 2
[0202] Embodiment 2: Preparation of LB80380 compound
[0203] step 1. Preparation of 1-(aminomethyl)cyclopropoxymethylphosphonic acid diethyl ester (VII-1)
[0204]
[0205] Add 1-(dibenzylamino)methylcyclopropyloxymethylphosphonic acid diethyl ester (I-1) (80.0g, 0.2mol) and methanol (1.2L) into the autoclave, add 5%Pd / C (8.0g), catalytic hydrogenation after gas replacement (pressure 0.3MPa, temperature 25°C). The completion of the reaction was monitored by TLC, filtered, and the filtrate was concentrated under reduced pressure and purified by flash column chromatography (dichloromethane:methanol=5:1) to obtain an oily substance, a total of 41.36g, with a yield of 91%.
[0206] HNMR (400MHz, CDCl 3 )δ4.10-4.17(m,4H),3.78(d,2H),2.78(s,2H),2.70(brs,2H),1.31(t,6H),0.88(t,2H),0.53(t ,2H).
[0207] Step 2. Diethyl (1-(2,5-diamino-6-chloropyrimidine-4-amino)methyl)cyclopropoxymethylphosphonate Preparation of (VIII-1)
[0208]
[0209] Diethyl 1-(aminomethyl)cyclop...
Embodiment 3
[0227] Embodiment 3: the preparation of intermediate compound I-1
[0228] step 1. Preparation of ethyl 2-(dibenzylamino)acetate (IV-1)
[0229]
[0230] Dissolve ethyl glycine (10.3g, 0.1mol) in dichloromethane (50ml), add triethylamine (20.2g, 0.2mol) at 25°C, and add benzyl chloride (25.32g, 0.2mol) under stirring , After the addition, a white solid precipitated out. Continue to stir, TLC monitors that the reaction is complete, filter, the filter cake is washed with dichloromethane (20ml), the combined filtrate is washed with water (100ml), the organic layer is dried over anhydrous sodium sulfate, filtered, concentrated to obtain a solid, and washed with petroleum ether / ethyl acetate (100:1) recrystallization to obtain 27.21 g of white solid with a yield of 96.03%.
[0231] HNMR is the same as that in Step 1 of Example 1.
[0232] Step 2. Preparation of 1-(dibenzylamino)methylcyclopropanol (II-1)
[0233] Prepare with reference to step 2 of Example 1.
[0234] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com