Novel thiol compound and polymerizable composition containing same
A technology of polymeric composition and thiol compound, which is applied in thiol preparation, organic chemistry, instruments, etc., can solve the problems of high production cost, reduced reactivity, and increase in production cost of optical materials, and achieve the effect of saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
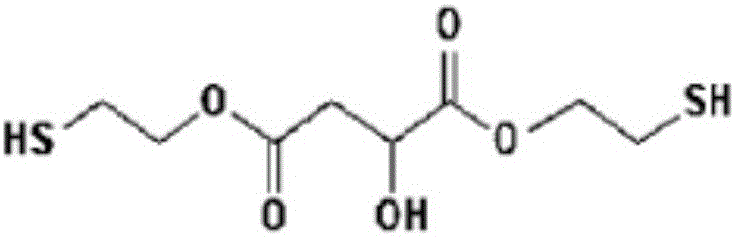
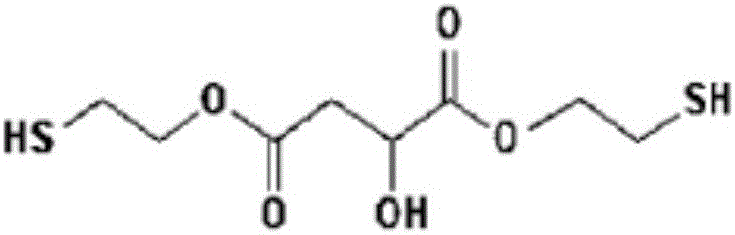
[0051] BMHS1 (bis(2-mercaptoethyl)-2-hydroxysuccinate (bis(2-mercaptoethyl)-2- hydroxysuccinate))
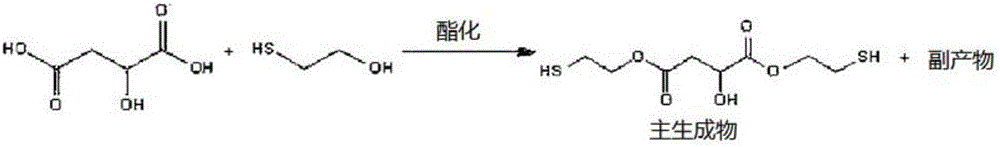
[0052] Install a Dean-Stark (Dean-stark) device in a 1L reactor with adjustable temperature, and add malic acid (134.09g, 1.00 mole), 2-mercaptoethanol (156.26g, 2.00 mole), 130g Toluene and p-toluenesulfonic acid (11.41 g, 0.06 mol) were stirred and reacted for 7 hours while maintaining a reduced pressure of 100 Torr and an internal temperature of 50°C. The reaction was performed while removing water generated during the reaction using a Dean-Stark apparatus. The reaction was terminated when the amount of theoretically produced water was 36 g and 2-mercaptoethanol as a starting material by high performance liquid chromatography (HPLC) disappeared. If the reaction ends, then reduce the temperature of the reactor, when reaching room temperature, in order to remove residual p-toluenesulfonic acid, utilize 200g of NaHCO of 5 weight percent (wt%) 3 (aq) Slowly carry out neutra...
Synthetic example 2
[0054] BMHS2 (bis(2-mercaptoethyl)-2-hydroxysuccinate (bis(2-mercaptoethyl)-2- hydroxysuccinate))
[0055] Install the Dean-Stark device in a 1L reactor with adjustable temperature, and add malic acid (134.09g, 1.00 mole), 2-mercaptoethanol (160.20g, 2.05 mole), 200g of toluene, p- Toluenesulfonic acid (11.41 g, 0.06 mol) was stirred and reacted for 7 hours while maintaining a reduced pressure state of 100 torr and an internal temperature of 50°C. The reaction was carried out while removing water generated during the reaction from the reactants using a Dean-Stark apparatus. The reaction was terminated when the theoretical amount of water and 2-mercaptoethanol as a starting material obtained by high performance liquid chromatography disappeared. If the reaction ends, then reduce the temperature of the reactor, when reaching room temperature, in order to remove residual p-toluenesulfonic acid, utilize 200g of NaHCO of 5 weight percent 3 (aq) Slowly carry out neutralization...
Embodiment 1
[0059] The compound (BMHS1) prepared in the above Synthesis Example 1, 53.37g of 2,5(6)-bis(isocyanatomethyl)bicyclo[2.2.1]heptane (NBDI), as a release agent 0.12 g of Zelec UN, 1.50 g of 2-(2'-hydroxy-5-methylphenyl)-2H-benzotriazole as UV absorber, 0.05 dibutyl dichloride as polymerization initiator Tin, 20ppm of 1-hydroxyl-4-p-toluidine) anthraquinone (Blue), 10ppm of perionone dyes as organic dyes are put into the matching barrel with agitator installed, and nitrogen is used to replace air, thereby removing Match the air in the barrel. The mixed solution was defoamed for 1 hour under the condition of 1 torr. Thereafter, it was filtered through a filter made of 1 μm polytetrafluoroethylene (PTFE, Polytetrafluoroethylene), and poured into a glass mold fixed with adhesive tape. This mold was put into a polymerization oven, and the temperature was gradually raised to 25° C. to 130° C. over 21 hours to perform polymerization. After the polymerization was complete, the mold w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com