Pharmaceutical composition for conducting infusion therapy in bladder lumen
A technology for treating drugs and compositions, applied in the field of carriers of low water-soluble drugs, can solve the problems of low bladder wall permeability, ectopic ventricular rhythm, aggravating the burden of liver and kidney functions, etc. The effect of reducing the amount of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] Prescription composition:
[0034] Chitosan
[0035] sodium triphosphate
[0036] Thioglycolic acid
[0037] Gemcitabine Hydrochloride
[0038] Preparation:
[0039] Add 1.5g of chitosan to 100ml of thioglycolic acid and 1g of concentrated sulfuric acid, stir in a water bath at 40°C for 24h, filter, wash with distilled water and ethanol, and dry in vacuum at 35°C to generate mercaptochitosan.
[0040] 2. Mercaptochitosan was dissolved in distilled water (2 mg / ml), and the pH was adjusted to 4.8; the concentration of sodium triphosphate aqueous solution was 1 mg / ml, and gemcitabine hydrochloride was dissolved in sodium triphosphate solution (5 mg / ml); stirred at room temperature The mercapto-chitosan solution was added dropwise to the sodium triphosphate solution in which gemcitabine was dissolved, then centrifuged for 1 hour at a speed of 13500 rpm, and the supernatant was discarded to generate chitosan particles encapsulated with gemcitabine.
[0041] 3. According...
example 2
[0043] Prescription composition:
[0044] Oxybutynin Hydrochloride
[0045] Chitosan
[0046] Hydroxypropylmethylcellulose
[0047] Poloxamer 407
[0048] Preparation:
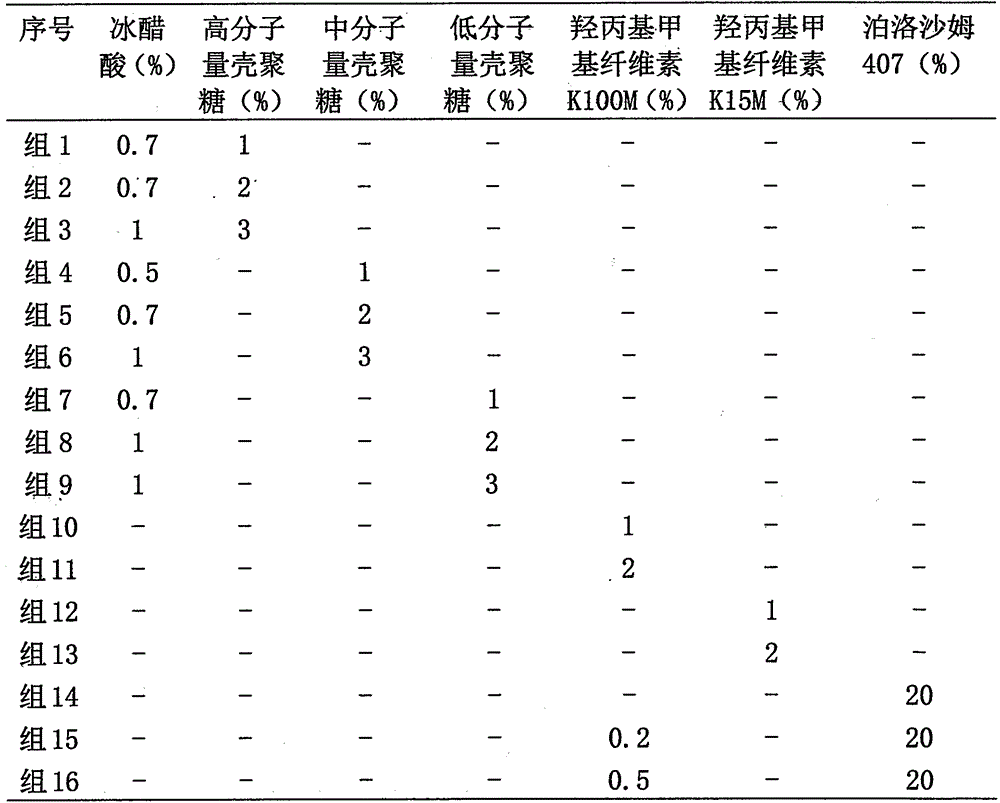
[0049] Method 1 (group 1-9): glacial acetic acid was dissolved in half an amount of distilled water, and chitosan was added according to the percentage by weight shown in Table 1, and stirred slowly; Oxybutynin hydrochloride was dissolved in half an amount of distilled water; at room temperature, two The two solutions were mixed and stirred at a stirring rate of 500 rpm until a uniform gel was formed. Then add base, other additives and water as needed.
[0050] Method 2 (groups 10-13): at room temperature, add hydroxypropyl methylcellulose into distilled water dissolved in oxybutynin hydrochloride according to the weight percentage shown in Table 1, stir for 1 hour, and the speed is 500rpm, Generate target gels. Then add base, other additives and water as needed.
[0051] Method 3 (group 14-16): at 4°C...
example 3
[0055] Prescription composition:
[0056] Paclitaxel
[0057] ethyl acetate
[0058] polyvinyl alcohol
[0059] Polymethacrylate
[0060] Preparation method: 50 mg of polymethacrylate and 2.5 mg of paclitaxel were dissolved in 1.5 ml of ethyl acetate; the above solution was added into 15 ml of 2% polyvinyl alcohol solution by mass percentage, and emulsified for 5 minutes. The above emulsion was stirred at room temperature until the ethyl acetate was completely evaporated. Separate target microspheres, clear with distilled water, centrifuge at 4000rpm for 5 minutes, repeat 3 times; store in 4°C environment in the form of water suspension. Then add base, other additives and water as needed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com