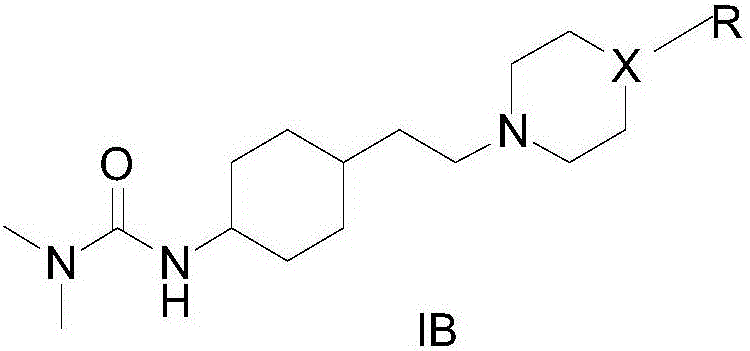
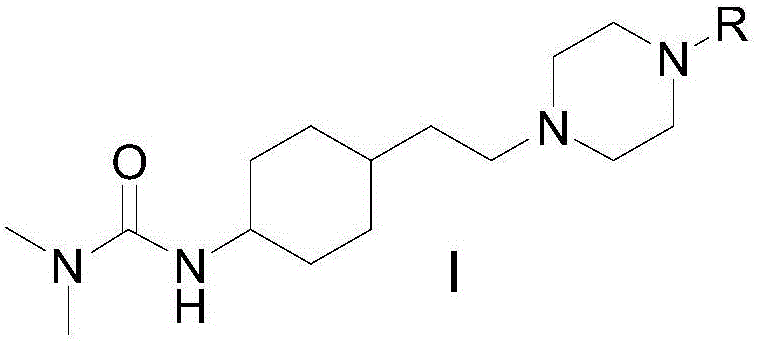
Cyclohexane derivative or stereoisomer or salt and preparation and application thereof
一种立体异构体、衍生物的技术,应用在药物化学领域,能够解决不能满足精神类疾病治疗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Preparation of 1-benzo[b]thiophene-4-piperazine hydrochloride
[0094]
[0095]7.20g 4-bromobenzo[b]thiophene, 19.9g piperazine anhydride, 4.70g sodium tert-butoxide, 0.32g (R)-(+)-2,2′-bis(diphenylphosphino)- A mixture of 1,1'-binaphthalene (BINAP), 0.63 g of dipalladium tris(dibenzylideneacetone) and 150 ml of toluene was refluxed for 1 hour under a nitrogen atmosphere. Pour 150ml of water into the reaction solution, then extract with 100ml×3 ethyl acetate, wash with water, dry over anhydrous magnesium sulfate, and evaporate the solvent under reduced pressure (0.01MPa, 45°C). The residue was purified by silica gel column chromatography (dichloromethane:methanol:25% aqueous ammonia=100:10:1) to obtain 4.60 g of 1-benzo[b]thiophen-4-yl-piperazine as a yellow oil. 2ml of concentrated hydrochloric acid was added to a methanol solution (25ml) containing 4.6g of 1-benzo[b]thiophen-4-yl-piperazine, and the solvent was evaporated under reduced pressure (0.01MPa, 45°C). E...
Embodiment 2
[0097] Preparation of trans-4-[2-[4-(benzo[b]thiophene)-7-piperazinyl]ethyl]cyclohexyl-carbamic acid tert-butyl ester
[0098]
[0099] 2.54g (10mmol) 1-benzo[b]thiophene-4-piperazine hydrochloride (prepared in Example 1) and 2.40g (10mmol) trans-2-{1-[4-(N-tert Butoxycarbonyl)amino]cyclohexyl}-acetaldehyde was dissolved in 120ml of dichloromethane, and at room temperature (25°C±2°C), 1.40ml (10mmol) of triethylamine was added and stirred slowly for 10 minutes, then gradually added 3.16g (14.8mmol) sodium triacetoxyborohydride, stirring reaction was continued at room temperature for 24 hours, after the reaction was completed, 120ml of 10% sodium bicarbonate solution was added. The reaction system was directly extracted and separated, the organic phase was dried with anhydrous sodium sulfate, and finally filtered and rotary evaporated to dryness, the solid was refluxed with 15ml of ethyl acetate and cooled to room temperature (25°C±2°C) for crystallization to obtain 3.70g of...
Embodiment 3
[0102] Preparation of trans-4-[2-[4-(benzo[b]thiophene)-7-piperazinyl]ethyl]cyclohexylamine
[0103]
[0104] Under ice-water bath, 4.43g trans-4-[2-[4-(benzo[b]thiophene)-7-piperazinyl]ethyl]cyclohexyl-carbamic acid tert-butyl ester (prepared in Example 2 ) was placed in a reaction flask, 80ml of saturated hydrogen chloride in ethyl acetate was added, stirred and reacted for 8 hours to carry out the deprotection reaction, and finally a white precipitate was formed to obtain 3.42g of the hydrochloride of the title compound. The above solid was added to 50ml of dichloromethane solution, and 50ml of saturated sodium bicarbonate solution was stirred for half an hour, then separated and extracted, and the organic phase was concentrated (0.01MPa, 40°C) to obtain 3.30g of the target product.
[0105] 1 H-NMR (CDCl 3 )δppm: 7.78 (1H, d, J = 5.5Hz), 7.76 (1H, d, J = 8.1Hz), 7.37 (1H, m), 7.29 (1H, d, 7.6Hz), 6.96 (1H, d, J=7.6Hz), 3.48-3.38(8H,m), 2.53(1H,m), 2.46(2H,m), 1.78-1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com