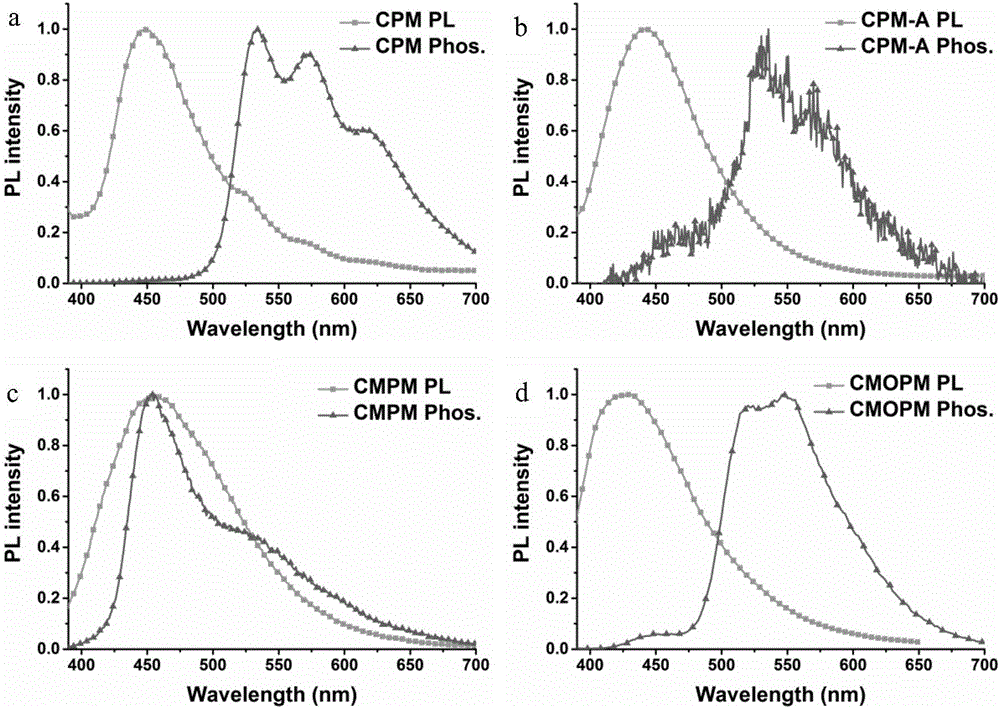
N-substituted carbazole-amide room temperature phosphorescent molecules and preparation method and application thereof
A room temperature phosphorescence and carbazole technology, which is applied in the directions of organic chemistry methods, chemical instruments and methods, luminescent materials, etc., can solve the problems of short lifespan of room temperature phosphorescence, and achieve the effects of long phosphorescence lifespan, simple post-processing, and simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of compound CPM
[0032]
[0033] Aluminum trichloride (1.33 g, 10 mmol) and carbon disulfide (10 mL) were added to the flask, and benzoyl chloride (1.2 mL, 10 mmol) was added dropwise with stirring. After stirring for 20 minutes, a solution of carbazole (0.836 g, 5 mmol) in carbon disulfide (40 mL) was added dropwise through a constant pressure dropping funnel, and the reactor was connected to an exhaust gas absorption device. After reacting at 40°C for three hours, dilute hydrochloric acid was slowly added dropwise to the flask to terminate the reaction, the reaction solution was extracted with dichloromethane (3×40mL), the organic phase was collected, and washed with anhydrous Na 2 SO 4 Dry and remove the solvent to give the crude product. A mixed solvent of petroleum ether and dichloromethane (v / v, 1 / 1) was used as an eluent, separated and purified by silica gel chromatography, and dried in vacuo to obtain a white solid (yield 30%)...
Embodiment 2
[0035] Embodiment 2: the preparation of compound CPM
[0036] Sodium hydride (0.34g, dispersed in mineral oil, content 70%, 9.9mmol) was added to the flask, then a solution of carbazole (0.835g, 5mmol) in DMF (20mL) was added, the reaction was stirred for half an hour, and benzyl was added dropwise Acid chloride (0.9 mL, 7.5 mmol) in DMF (10 mL) and the reaction was stirred overnight. Slowly add water to the reaction solution to terminate the reaction, the reaction solution was extracted with dichloromethane (3 × 40mL), washed with water several times to remove DMF, the organic phase was collected, and washed with anhydrous Na 2 SO 4 Dry and remove the solvent to give the crude product. Using a mixed solvent of petroleum ether and dichloromethane (v / v, 1 / 1) as an eluent, separate and purify using a silica gel column, and dry in vacuo to obtain CPM as a white solid.
Embodiment 3
[0037] Embodiment 3: the preparation of compound CMPM
[0038]
[0039] Sodium hydride (0.34g, dispersed in mineral oil, content 70%, 9.9mmol) was added to the flask, then a solution of carbazole (0.835g, 5mmol) in DMF (20mL) was added, and after stirring for half an hour, 4- Toluyl chloride (1 mL, 7.5 mmol) was dissolved in DMF (10 mL) and the reaction was stirred overnight. Slowly add water to the reaction solution to terminate the reaction, the reaction solution was extracted with dichloromethane (3 × 40mL), washed with water several times to remove DMF, the organic phase was collected, and washed with anhydrous Na 2 SO 4 Dry and remove the solvent to give the crude product. A mixed solvent of ethyl acetate and petroleum ether (v / v, 1 / 20) was used as an eluent, separated and purified by silica gel chromatography, and dried in vacuo to obtain a white solid (yield 49%). 1 H NMR, 13 C NMR, MS and EA characterize the structure, confirm that this white solid is compound C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com