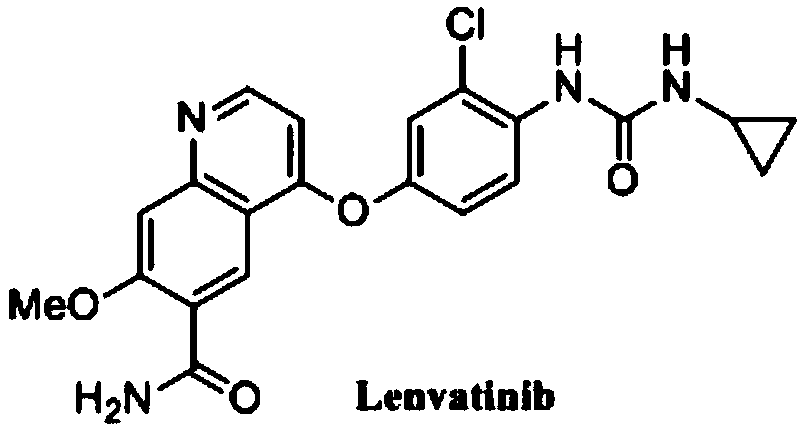
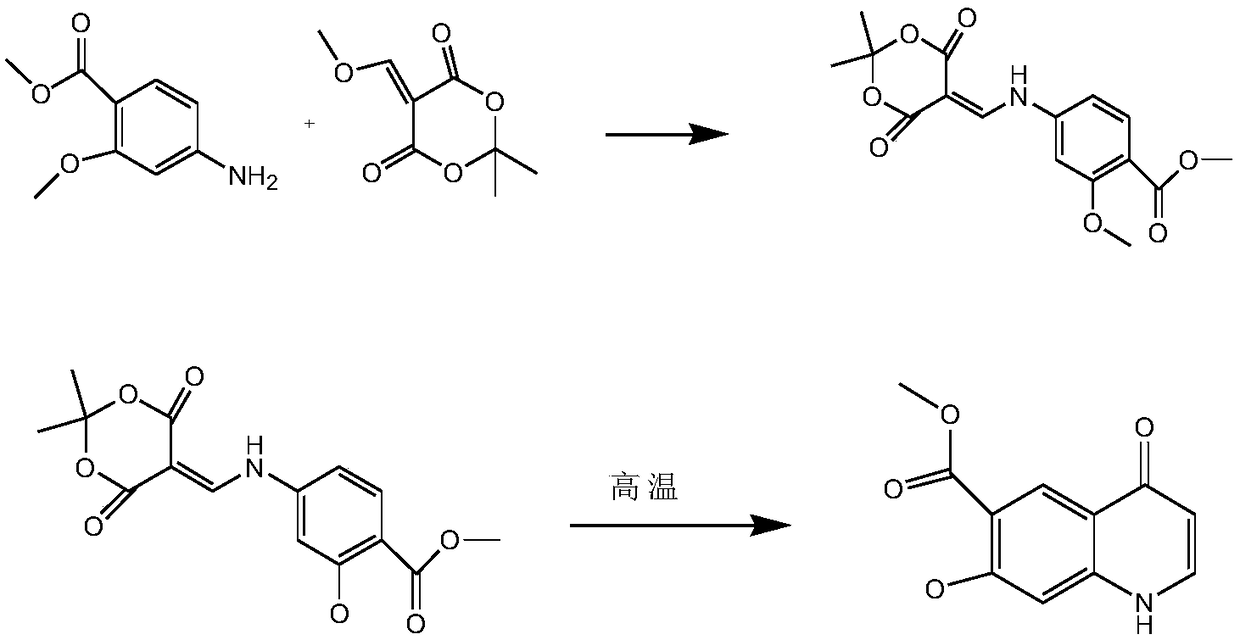
A kind of synthetic method of 4-oxo-7-methoxy-1,4-dihydroquinoline-6-carboxylic acid methyl ester
A technology of methyl methoxybenzoate and methyl nitrobenzoate, which is applied in the field of pharmaceutical synthesis and achieves the effects of being conducive to enlarged production, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
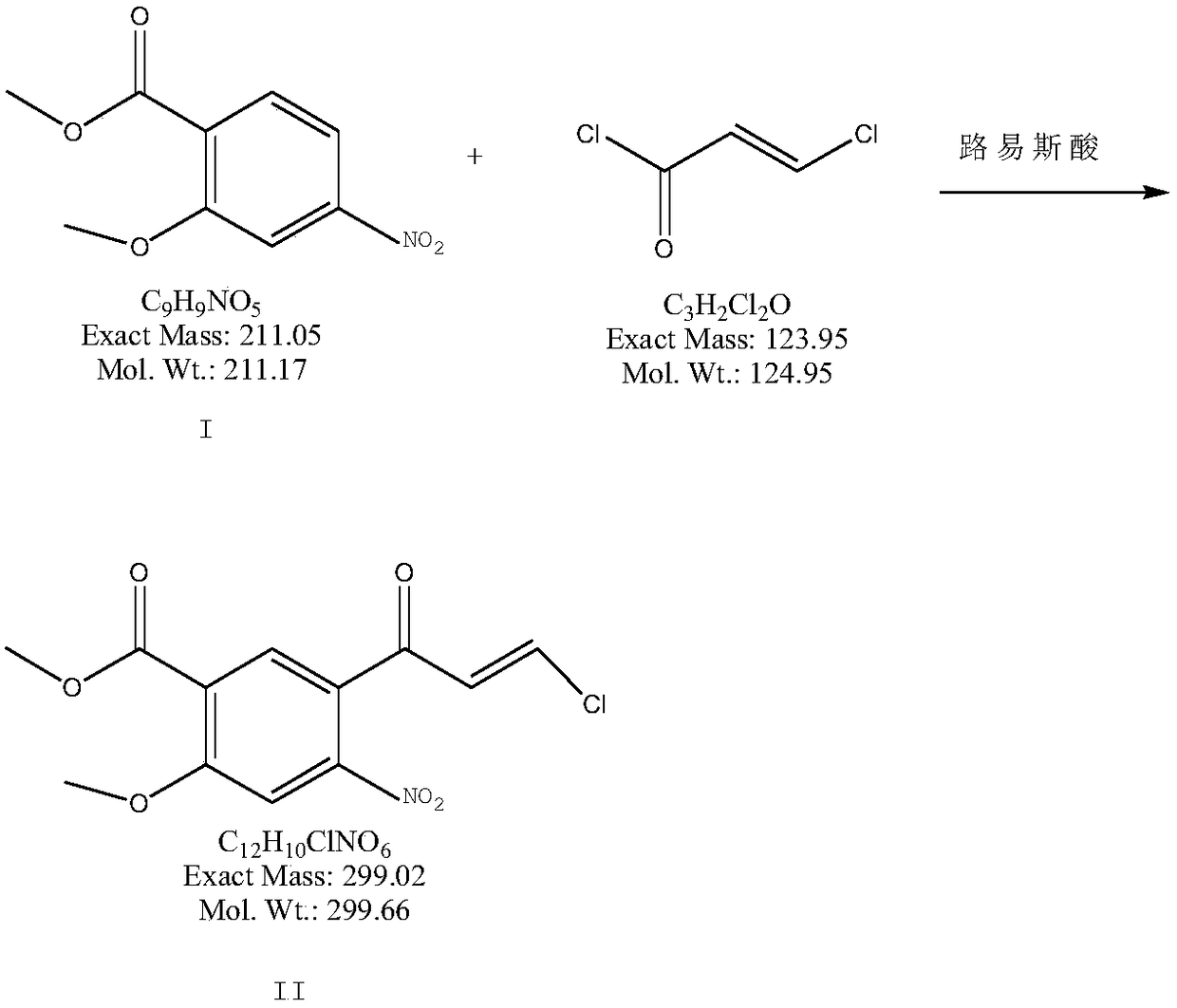
[0029] a) Put 2kg of toluene and 211g of methyl 2-methoxy-4-nitrobenzoate into the dry reaction flask, cool down to below 0°C, drop in 200g of anhydrous aluminum chloride, control the temperature at 0-5°C, and Add 137 grams of 3-chloroacryloyl chloride dropwise into the reaction bottle, slowly warm up to room temperature and stir for 16 hours after dropping, cool down to 0-5°C, pour the reaction solution into 2000ml of cold 6N hydrochloric acid, stir for 1 hour, pump The product was obtained by filtration, washed with purified water until neutral, and dried at 60°C to obtain 270 g of compound II with a yield of 90%.
[0030] b) Put compound II, 299g, into the reaction flask, add 2000g of 20% ethanol water, add 60g of ammonium chloride, control the internal temperature to be less than 45°C, add 160g of reduced iron powder in batches, and slowly raise the temperature to 85°C for reaction After 3 hours, the iron sludge was filtered while it was hot, and the mother liquor was cool...
Embodiment 2
[0033] a) In a dry reaction flask, 220 g of methyl 2-methoxy-4-nitrobenzoate is dropped into 2 kg of dichloromethane containing 183 g of boron trichloride, and the temperature is controlled at 0-5° C., and the 3- Add 169 grams of chloroacryloyl chloride dropwise into the reaction bottle, stir at room temperature for 12 hours after dropping, cool down to 0-5°C, pour the reaction solution into 2000ml of cold 6N hydrochloric acid, stir for 1 hour, filter the product with suction, and purify Washed with water until neutral, and dried at 60°C to obtain 281 g of compound II with a yield of 90.1%.
[0034] b) Put 299g of compound II into the reaction flask, add 2000g of 20% ethanol water, add 64g of ammonium chloride, control the internal temperature to be less than 45°C, add 163g of reduced zinc powder in batches, and slowly heat up to 80°C for reaction After 4 hours, it was filtered while it was hot, and the mother liquor was cooled to below 0°C to obtain the product by suction fil...
Embodiment 3
[0037] a) Put 3kg of chloroform and 232g of methyl 2-methoxy-4-nitrobenzoate into the dry reaction bottle, cool down to -10°C, put in 219g of anhydrous aluminum trichloride, and control the temperature at 0-25°C , Add 164 grams of 3-chloroacryloyl chloride dropwise into the reaction flask, stir at room temperature for 1.2 hours after dropping, slowly raise the temperature to 45°C and stir for 4.5 hours, cool down to 1°C, and pour the reaction solution into 2000ml of cold 6N hydrochloric acid , stirred for 1.2 hours, and the product was obtained by suction filtration, washed with purified water until neutral, and dried at 60°C to obtain 301 g of compound II with a yield of 91.4%.
[0038] b) Put 300g of compound II into the reaction flask, add 2000g of 20% ethanol water, add 62g of ammonium chloride, control the internal temperature to be less than 45°C, add 200g of sodium sulfide in batches, and slowly raise the temperature to 83°C after the addition is complete. Reaction 3.4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com