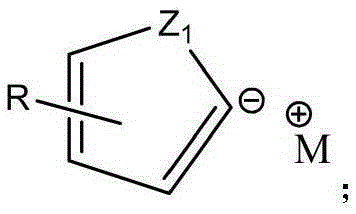
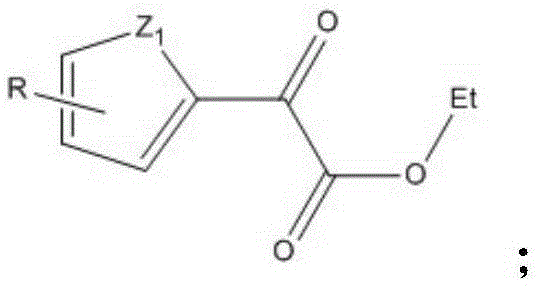
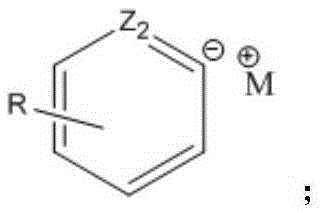
Industrial preparation method of heterocyclic derivative
A derivative and industrial technology, applied in the field of industrial preparation of heterocyclic derivatives, can solve the problems of lengthy synthetic routes, lower production efficiency, and higher production costs, and achieve the effects of increased operational difficulties, increased costs, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059]
[0060] Slowly add 700g of furan (tetrahydrofuran dissolved in 1000ml) dropwise into 230g of metal sodium sand (an electron transfer agent such as α-methylstyrene can also be added during the reaction), react at room temperature for 2-4 hours, and Add 1500g diethyl oxalate dropwise while stirring, react at room temperature for 2 hours, filter, neutralize the reaction solution to pH=6.5-7.5, extract twice with 500ml ethyl acetate, take the organic layer, evaporate the solvent to obtain 1519g product, the product The final ester compound can be obtained by reduction.
Embodiment 2
[0062]
[0063] Slowly add 900g of p-picoline dropwise to 400g of potassium metal, reflux for 1 hour, cool to room temperature, add 1400g of diethyl oxalate dropwise while stirring, reflux for 4 hours, heat filter, and neutralize the reaction solution to pH =6.5-7.5, extracted twice with 500ml ethyl acetate, took the organic layer, evaporated the solvent to obtain 1732g product.
[0064] In this reaction, the N on the pyridine ring can also be considered to be protected before proceeding.
Embodiment 3
[0066]
[0067] Slowly add 900g of 2-methylfuran dropwise to 1500g of tert-butyllithium, reflux for 4 hours, cool to room temperature, add 1460g of diethyl oxalate dropwise while stirring, reflux for 1 hour, and obtain 1701g of product by rectification .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com