High-yield oxapium iodide preparation method
A technology of oxalidium and high yield, which is applied in the field of preparation of high-yield oxalidium, can solve the problems of being unable to adapt to industrialized production, unsuitable for industrialized production, low yield of oxalidium, and the like, and achieves a technological The reaction time is short, the product purification method is simple, and the effect of increasing the comprehensive yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
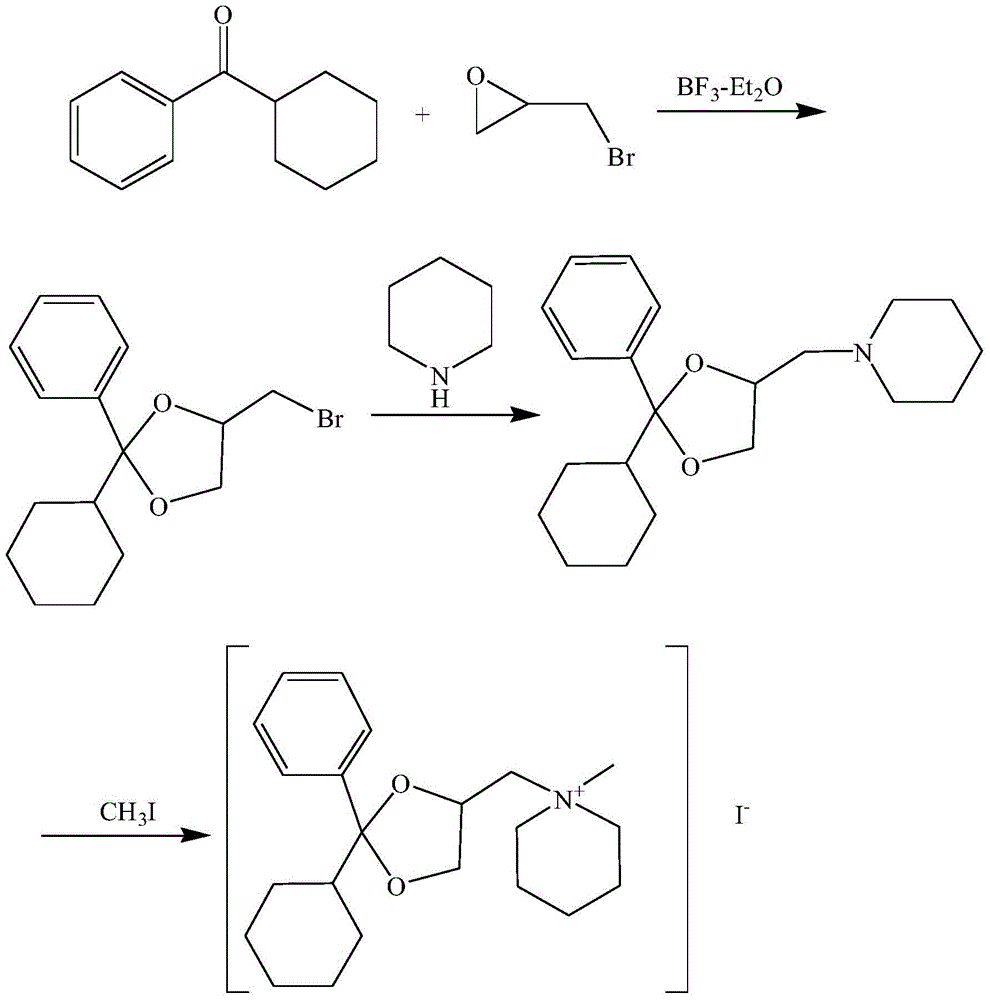
[0022] Reference figure 1 , The method for preparing oxalidomium with high yield provided by the present invention includes the following steps:
[0023] S1. Preparation of 2-phenyl-2-cyclohexyl-4-bromomethyl-1,3-dioxane: add boron trifluoride ether complex dropwise to dry cyclohexyl phenyl ketone, adjust Temperature, drip dry 1-bromo-2,3-epoxypropane, continuously stir during the dripping process, adjust the temperature, continue stirring, under reduced pressure distillation of boron trifluoride ether complex and cyclohexyl phenyl ketone to obtain Material A; Material A is purified and dried to obtain 2-phenyl-2-cyclohexyl-4-bromomethyl-1,3-dioxolene;
[0024] S2. Preparation of 2-phenyl-2-cyclohexyl-4-piperidinemethyl-1,3-dioxolone: the piperidine, the second organic solvent, the catalyst and the 2-phenyl-2- Mix and dissolve cyclohexyl-4-bromomethyl-1,3-dioxane; adjust the temperature to 25-35℃, keep stirring for 4-6h, and concentrate under reduced pressure to obtain material...
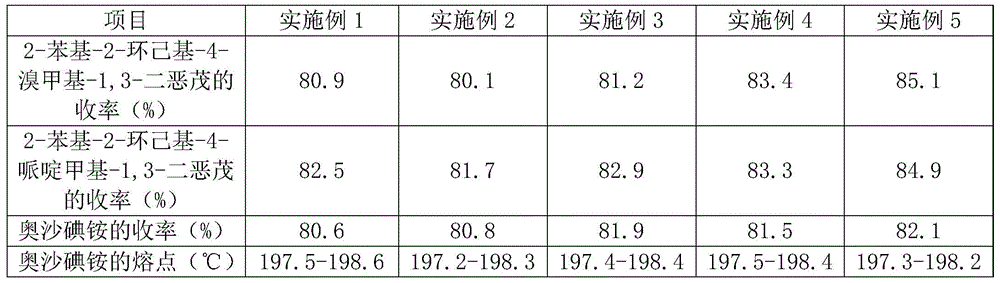
Embodiment 1
[0028] A method for preparing high-yield oxalinium ammonium includes the following steps:
[0029] S1. Preparation of 2-phenyl-2-cyclohexyl-4-bromomethyl-1,3-dioxane: Add 0.84 parts of boron trifluoride ether complex dropwise to 75 parts of dry cyclohexyl by weight In the phenyl ketone, adjust the temperature to 0°C, add dropwise 41 parts of 1-bromo-2,3-epoxypropane dry, stir continuously during the dropping process, adjust the temperature to 5°C, continue to stir for 7h, at 80 Distill boron trifluoride ether complex and cyclohexyl phenyl ketone under reduced pressure at a temperature of ℃ to obtain material A; add material A to ethanol to recrystallize and dry to obtain 2-phenyl-2-cyclohexyl-4-bromide Methyldioxane;
[0030] S2. Preparation of 2-phenyl-2-cyclohexyl-4-piperidinylmethyl-1,3-dioxolone: According to parts by weight, 51 parts of piperidine, 450 parts of ethanol, 2.7 parts of aluminum trichloride and S1 The obtained 32 parts of 2-phenyl-2-cyclohexyl-4-bromomethyl-1,...
Embodiment 2
[0033] A method for preparing high-yield oxalinium ammonium includes the following steps:
[0034] S1. Preparation of 2-phenyl-2-cyclohexyl-4-bromomethyl-1,3-dioxane: 1.14 parts of boron trifluoride ether complex are added dropwise to 56 parts of cyclohexyl by weight In the phenyl ketone, adjust the temperature to 5°C, add dropwise 27 parts of dry 1-bromo-2,3-epoxypropane, keep stirring during the dropping process, adjust the temperature to 15°C, continue to stir for 5h, at 90 Distill boron trifluoride ether complex and cyclohexyl phenyl ketone under reduced pressure at a temperature of ℃ to obtain material A; add material A to acetonitrile to recrystallize and dry to obtain 2-phenyl-2-cyclohexyl-4-bromide Methyldioxane;
[0035] S2. Preparation of 2-phenyl-2-cyclohexyl-4-piperidinylmethyl-1,3-dioxolone: 34 parts of piperidine, 750 parts of ethanol, 1.7 parts of ferric chloride and S1 The obtained 65 parts of 2-phenyl-2-cyclohexyl-4-bromomethyl-1,3-dioxane were mixed and dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com