Acellular biological dermal material, and preparation method and application thereof
A decellularized and biological technology, applied in the field of decellularized dermal materials and their preparation, can solve the problems of shortage of tissue sources, high price, application limitations, etc., and achieve the effects of low cost, easy operation, good toughness and strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
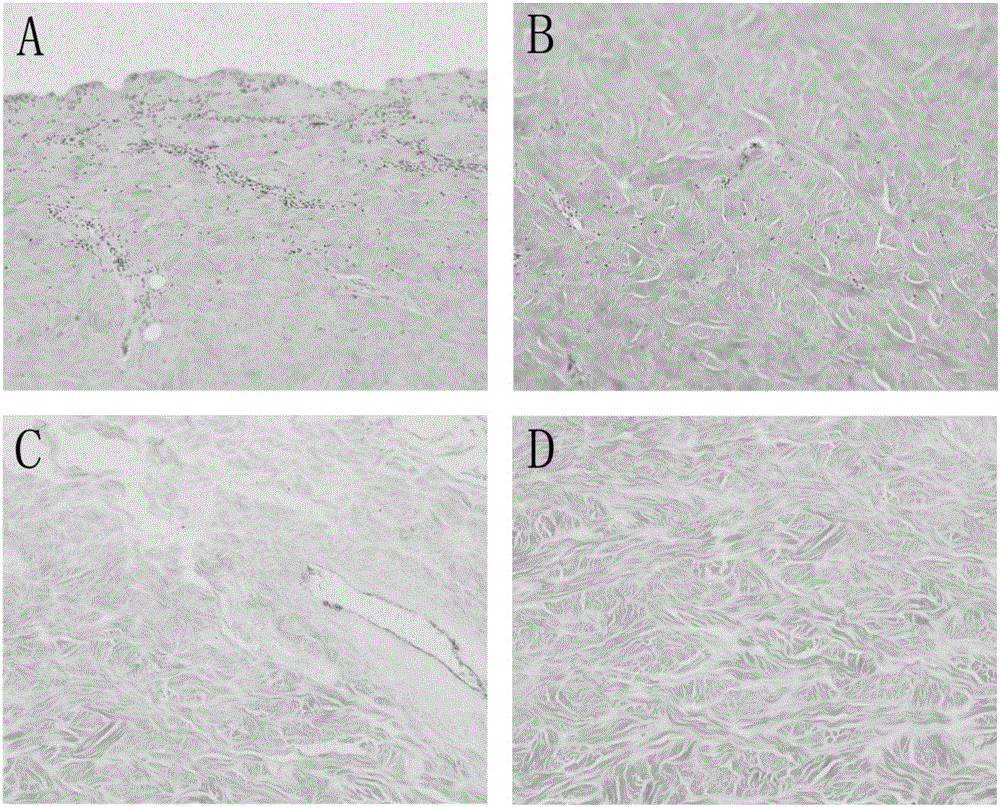
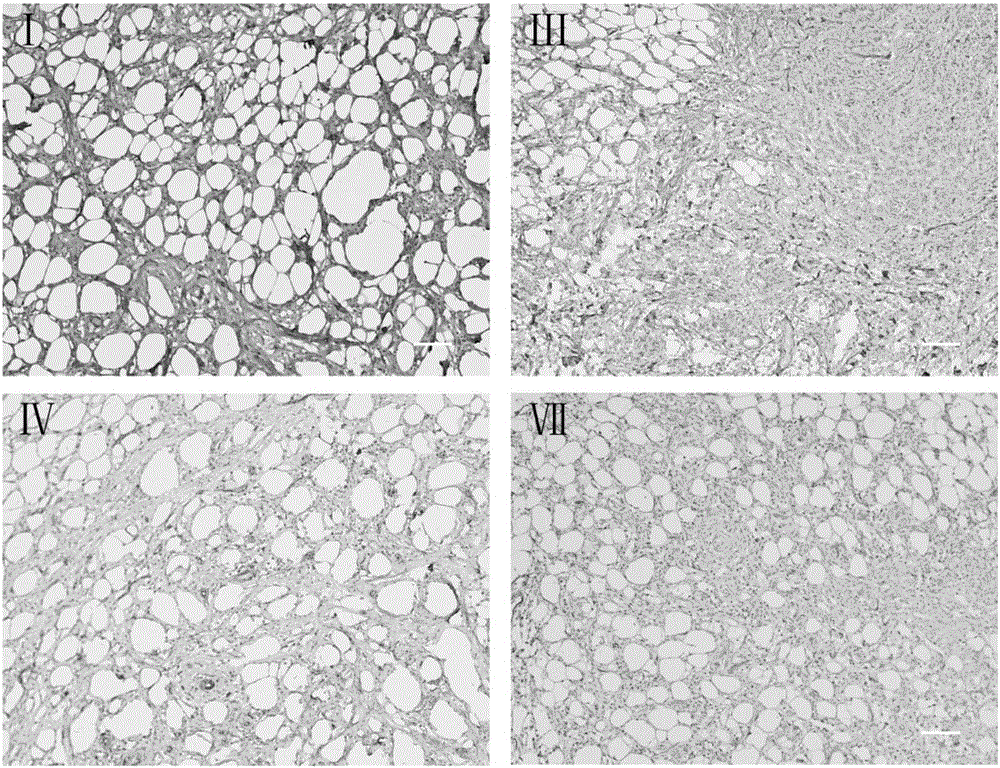
[0044] Example 1: Preparation of decellularized biological dermis material.
[0045] The full-thickness skin of the flank wall and back of a healthy adult pig was taken, cleaned and disinfected, cut into an appropriate size, disinfected and rinsed with 10mM Tris-Hcl containing 0.5wt% sodium phosphate and pH 7.2 aqueous solution, and then heated at 37°C with 0.02wt% EDTA and 0.15wt% trypsin (product number T4799 | CAS No. 9002-07-7 | SIGMA) and pH 7.4 sodium phosphate buffer soaked for 24 hours, the skin piece was taken out, and added with 0.5wt % sodium phosphate 10mM Tris-HCl and pH 7.2 aqueous solution for washing. With ionic detergent 0.1wt% TritonX-200 and 0.03wt% sodium lauryl sulfate and pH is 7.2 sodium phosphate buffer solution room temperature vibration soaking for 20 hours, further with 10mM Tris-HCl added with 0.5wt% sodium phosphate And the pH is 7.2 aqueous solution washing. Treat with 680U / L neutral protease (Dispase II product number: 04942078001|Roche) in 10m...
Embodiment 2
[0046] Example 2: Preparation of decellularized biological dermis material.
[0047] Take the full-thickness skin of the flank wall and back of a healthy adult pig, cut it into an appropriate size after cleaning and disinfection, disinfect and rinse it with 10mM Tris-Hcl containing 0.9wt% sodium chloride and a pH of 6.7 aqueous solution, and then heat it at 30°C. There are 0.03wt% EDTA and 0.3wt% trypsin (product number T4799 | CAS No. 9002-07-7 | SIGMA) and pH is 6.5 sodium phosphate buffer solution for 12 hours, the skin piece is taken out, and added with 0.9 Wash with 10 mM Tris-Hcl of wt% sodium chloride and pH 6.7. With 0.03wt% TritonX-200 and 0.08wt% sodium lauryl sulphate of ionic scale remover and pH is the sodium phosphate buffer of 6.7 and soaks for 18 hours at room temperature shaking, further adds 10mM Tris- with the sodium chloride of 0.9wt% Wash with Hcl and pH 6.7 aqueous solution. Add the 10mM Tris-Hcl of the sodium chloride of 0.9wt% with the dispase (Dispas...
Embodiment 3
[0048] Example 3: Preparation of decellularized biological dermis material.
[0049] The full-thickness skin of the flank wall and back of a healthy adult pig was taken, cleaned and disinfected, cut into an appropriate size, disinfected and rinsed with 10mM Tris-Hcl containing 0.7wt% sodium chloride and pH 7.8 aqueous solution, and then heated at 34°C There are 0.03wt% EDTA and 0.2wt% trypsin (product number T4799 | CAS No. 9002-07-7 | SIGMA) and pH is 7.8 Sodium phosphate buffer soaking treatment for 18 hours, the skin piece was taken out, and added with 0.7 The 10mM Tris-Hcl of wt% sodium chloride and the pH is 7.8 aqueous solution rinses. Sodium phosphate buffer solution with 0.03wt% TritonX-200 and 0.03wt% sodium lauryl sulfate and pH of 0.03wt% TritonX-200 ion scale remover and pH is soaked at room temperature for 24 hours, and further added with 10mM Tris- Wash with Hcl and pH 7.8 aqueous solution. Use 600U / L neutral protease (Dispase II product number: 04942078001|Roc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com