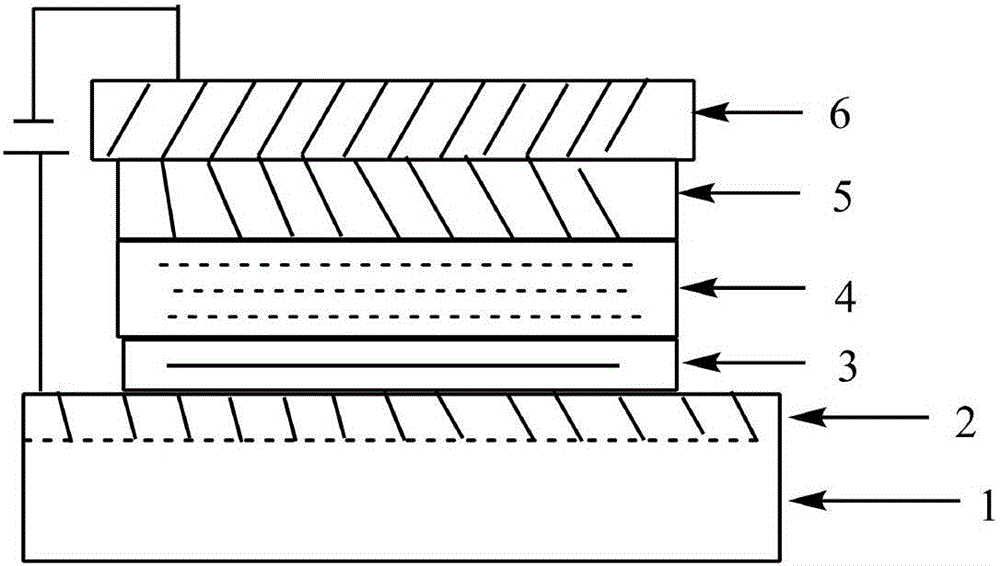
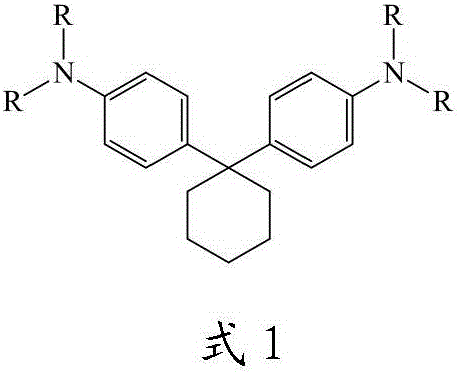
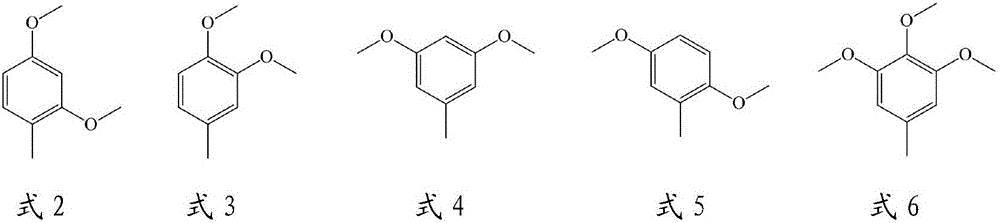
1,1-bis(4'-aminophenyl)cyclohexane group-containing hole transport material, preparation method and applications thereof
A technology of hole transport materials and cyclohexane groups, which is applied in the preparation of amino hydroxyl compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of poor thermal stability and low HOMO value, and achieve high yield , good solubility, high glass transition temperature and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of embodiment one compound 1
[0065] The reaction scheme is as follows:
[0066]
[0067] Add 2.66g (0.01mol) 1,1-bis(4-aminophenyl) cyclohexane, 9.75g (0.045mol) 2,4-dimethoxybromobenzene, 5.60g (0.05mol) Potassium tert-butoxide, 53.2 g toluene. Add 0.366g (4.0×10 -4 mol)Pd 2 (dba) 3 , 0.232g (8.0×10 -4 mol)(t-Bu) 3 PH·BF 4 , the temperature was raised to 100° C. to 108° C. for reflux reaction for 8 hours. After the reaction was completed, the reaction liquid was filtered, and the filter cake was rinsed with 20 g of toluene. The filtrate was passed through a silica gel column, and the solvent was removed under reduced pressure to obtain a reddish-brown viscous liquid. The above crude product was recrystallized by THF and toluene (mass ratio 3:3) to obtain 6.1 g of off-white solid, yield: 74.9%. LC-MS: [M] + = 691.46. 1 H-NMR(500MHz,d6-DMSO)δ / ppm: 7.15~7.18(d,4H),6.65~6.67(d,4H),6.51~6.53(d,4H),6.40~6.45(m,8H), 3.93(s,24H), 2.14~2.17(t,4H...
Embodiment 2
[0068] The preparation of embodiment two compound 2
[0069] The reaction scheme is as follows:
[0070]
[0071] Add 3.00g (0.011mol) 1,1-bis(4-aminophenyl) cyclohexane, 11.00g (0.051mol) 3,4-dimethoxybromobenzene, 6.32g (0.056mol) to a 250mL three-necked flask Potassium tert-butoxide, 60.0 g toluene. Add 0.412g (4.5×10 -4 mol)Pd 2 (dba) 3 , 0.261g (9.0×10 -4 mol)(t-Bu) 3 PH·BF 4 , the temperature was raised to 100° C. to 108° C. for reflux reaction for 8 hours. After the reaction was completed, the reaction solution was filtered, and the filter cake was rinsed with 25 g of toluene. The filtrate was passed through a silica gel column, and the solvent was removed under reduced pressure to obtain a reddish-brown viscous liquid. The above crude product was recrystallized through THF and toluene (mass ratio 3:3) to obtain 6.9 g of off-white solid, yield: 75.2%. LC-MS: [M] + = 691.46. 1 H-NMR(500MHz,d6-DMSO)δ / ppm: 7.15~7.17(d,4H),6.73~6.76(d,4H),6.65~6.67(d,4H),6.17~...
Embodiment 3
[0072] The preparation of embodiment three compound 3
[0073] The reaction scheme is as follows:
[0074]
[0075]Add 2.41g (9.0×10 -3 mol) 1,1-bis(4-aminophenyl)cyclohexane, 8.84 g (0.041 mol) 3,5-dimethoxybromobenzene, 5.08 g (0.045 mol) potassium tert-butoxide, 48.2 g toluene. Add 0.331g (3.6×10 -4 mol)Pd 2 (dba) 3 , 0.210g (7.2×10 -4 mol)(t-Bu) 3 PH·BF 4 , the temperature was raised to 100° C. to 108° C. for reflux reaction for 8 hours. After the reaction was completed, 50 g of water was added to wash twice, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a brown viscous liquid. The above crude product was recrystallized through THF and ethyl acetate (mass ratio 5:5) to obtain 5.6 g of off-white powder, yield: 76.8%. LC-MS: [M] + =811.41. 1 H-NMR (500MHz,d6-DMSO)δ / ppm: 7.15~7.17(d,4H),6.65~6.67(d,4H),5.74(s,12H),3.93(s,24H),2.14~2.17( t,4H), 1.52~1.56(t,6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com