Synthetic method of fluorine-containing alkyl substituted 2,3-dihydrocoumarone derivatives and indole derivatives
A technology of furan derivatives and indole derivatives, which is applied in the direction of organic chemistry and the like, achieves the effects of simple operation, mild reaction conditions and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 25 ml sealed tube reaction flask, add 0.15 mmol 2-allylphenol, 0.3 mmol ethyl iododifluoroacetate, 0.0075 mmol tetrakistriphenylphosphine palladium, 0.015 mmol 1,1'-bis (Diphenylphosphino)ferrocene, 0.3 mmol of cesium carbonate, 2 ml of toluene, the reaction system was stirred at 90° C. for 19 hours, stopped heating and stirring, and cooled to room temperature. Ethyl acetate extracted the reaction solution, decompressed rotary evaporation to remove the solvent, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a volume ratio of 100:1 petroleum ether: ethyl acetate mixed solvent , the yield was 71%.
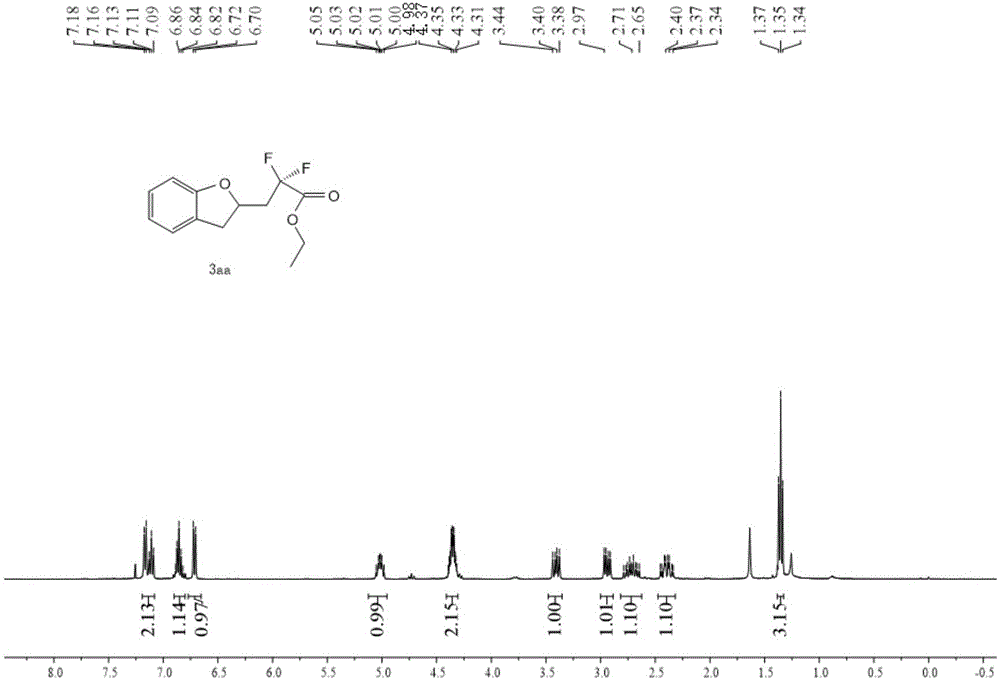
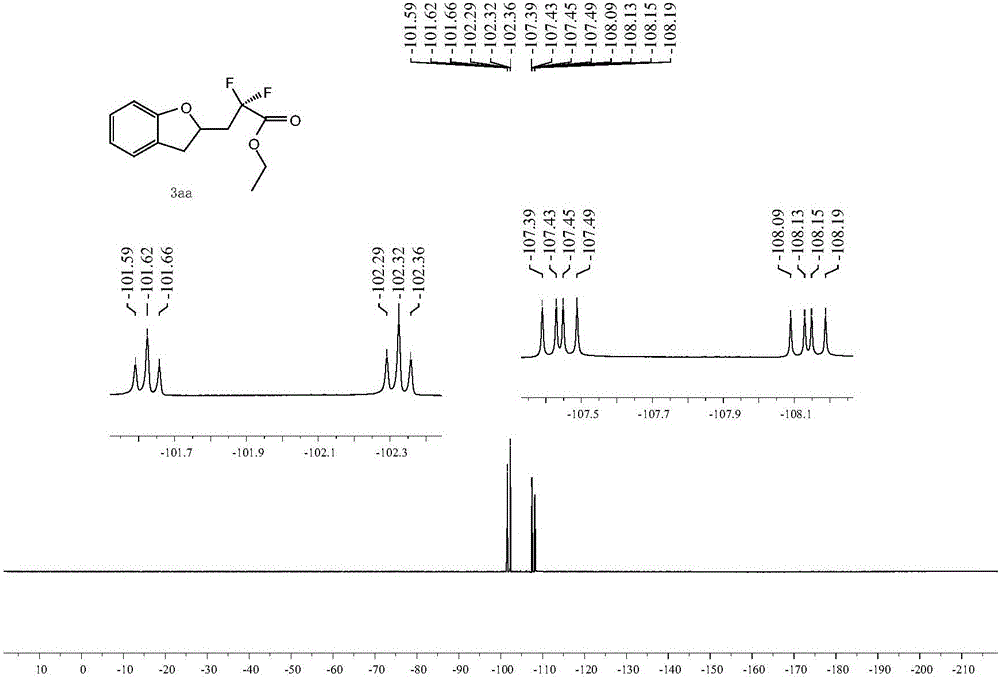
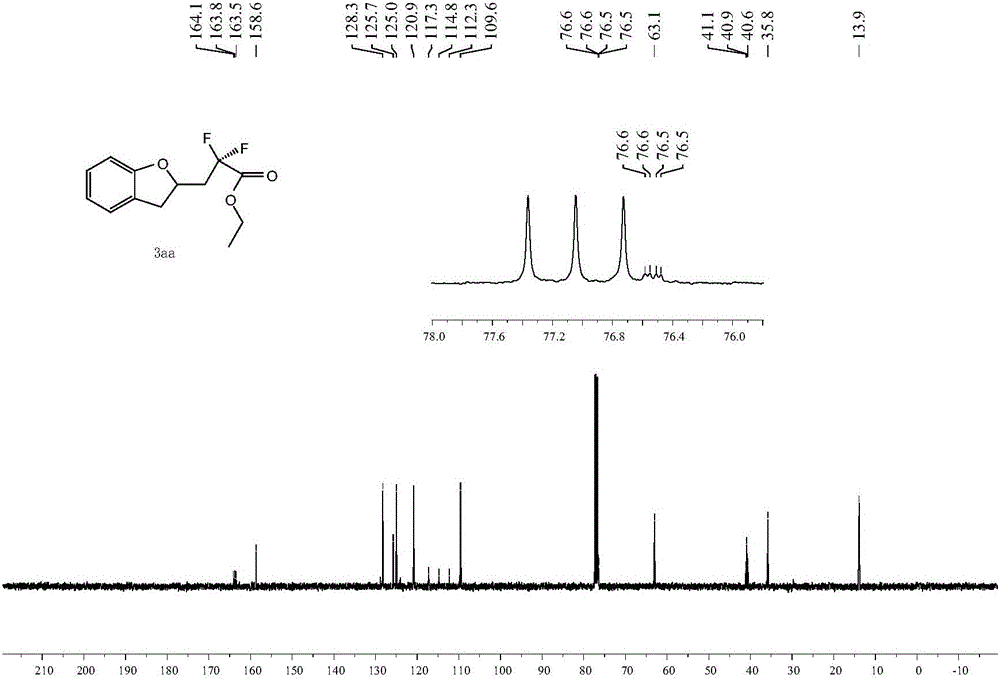
[0036] The proton nuclear magnetic spectrum, the fluorine spectrum and the carbon spectrum of the product obtained in this embodiment are respectively as follows figure 1 , figure 2 with image 3 As shown, the structural characterization data are as follows:
[0037] 1 H NMR (400MHz, CDC...
Embodiment 2
[0041] In a 25 ml sealed tube reaction flask, add 0.15 mmol 4-methyl 2-allylphenol, 0.3 mmol ethyl iododifluoroacetate, 0.0075 mmol tetrakistriphenylphosphine palladium, 0.015 mmol bis( Diphenylphosphorus)-1,1'-binaphthyl, 0.3 mmol potassium carbonate, 2 ml 1,4-dioxane, the reaction system was stirred at 90°C for 20 hours, stopped heating and stirring, and cooled to room temperature. Ethyl acetate extracted the reaction solution, decompressed rotary evaporation to remove solvent, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a volume ratio of 100:1 petroleum ether: ethyl acetate mixed solvent , the yield was 60%.
[0042] The structural characterization data of the product obtained in this embodiment are as follows:
[0043] 1 H NMR (400MHz, CDCl 3 )δ6.97(s,1H),6.90(d,J=8.1Hz,1H),6.60(d,J=8.1Hz,1H),5.02-4.95(m,1H),4.39-4.31(m,2H ), 3.36(dd, J=9.0Hz, J=15.6Hz, 1H), 2.89(dd, J=7.0Hz, J=15.6Hz,...
Embodiment 3
[0047] In a 25 ml sealed tube reaction flask, add 0.15 mmol 4-bromo-2-allylphenol, 0.3 mmol ethyl iododifluoroacetate, 0.0075 mmol dichlorodiacetonitrile palladium, 0.015 mmol 1,1 '-bis(diphenylphosphino)ferrocene, 0.3 mmol potassium acetate, 2 ml 1,4-dioxane, the reaction system was stirred and reacted at 80°C for 20 hours, stopped heating and stirring, and cooled to room temperature. Ethyl acetate extracted the reaction solution, decompressed rotary evaporation to remove the solvent, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a volume ratio of 50:1 petroleum ether: ethyl acetate mixed solvent , the yield was 75%.
[0048] The structural characterization data of the product obtained in this embodiment are as follows:
[0049] 1 H NMR (400MHz, CDCl 3 )δ7.14-7.11(m, 1H), 6.51(d, J=8.5Hz, 1H), 4.96(ddd, J=5.0Hz, J=8.1Hz, J=15.9Hz, 1H), 4.33-4.21( m, 2H), 3.32(dd, J=9.1Hz, J=15.9Hz, 1H), 2.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com