A kind of dura mater patch, preparation method and application in repairing of dura mater injury
A dura mater and patch technology, which is applied in the field of tissue engineering of biomedical materials, can solve the problems of fluid leakage, unconsidered patch strength, elasticity, elongation, and low bursting strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
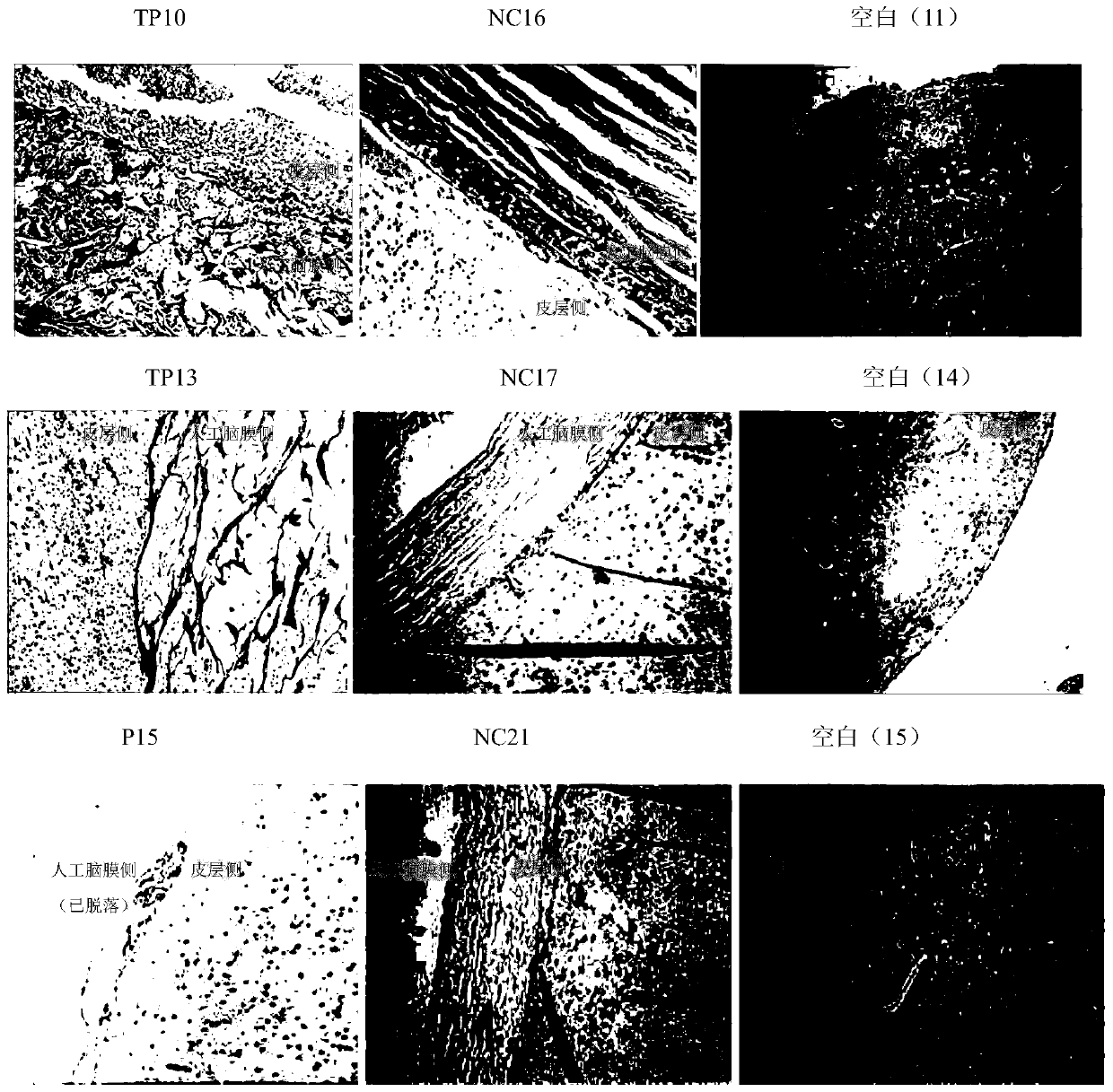
Embodiment 1
[0056] Example 1 Preparation method of acellular allogeneic dermal matrix
[0057] The present invention provides a method for preparing an acellular allogeneic dermal matrix, and a preferred preparation embodiment includes performing the following steps in order:
[0058] Put the raw materials into a container containing a cross-linking agent solution and soak for 4 hours. The cross-linking agent is genipin and N-hydroxysuccinimide, and the cross-linking agent is genipin and N-hydroxysuccinimide The mass ratio of the cross-linking agent to the allograft skin is 1.2:1, soaked for 8 hours. Take out the raw material, put it into another container filled with physiological saline solution, soak for 56 hours at 5°C, change the physiological saline solution every 8 hours, and obtain the semi-finished product A.
[0059] Put the semi-finished product A into 0.2% trypsin-dispase enzyme solution (w / v) for soaking and shaking, wherein the mass ratio of trypsin to dispase enzyme is 2:1...
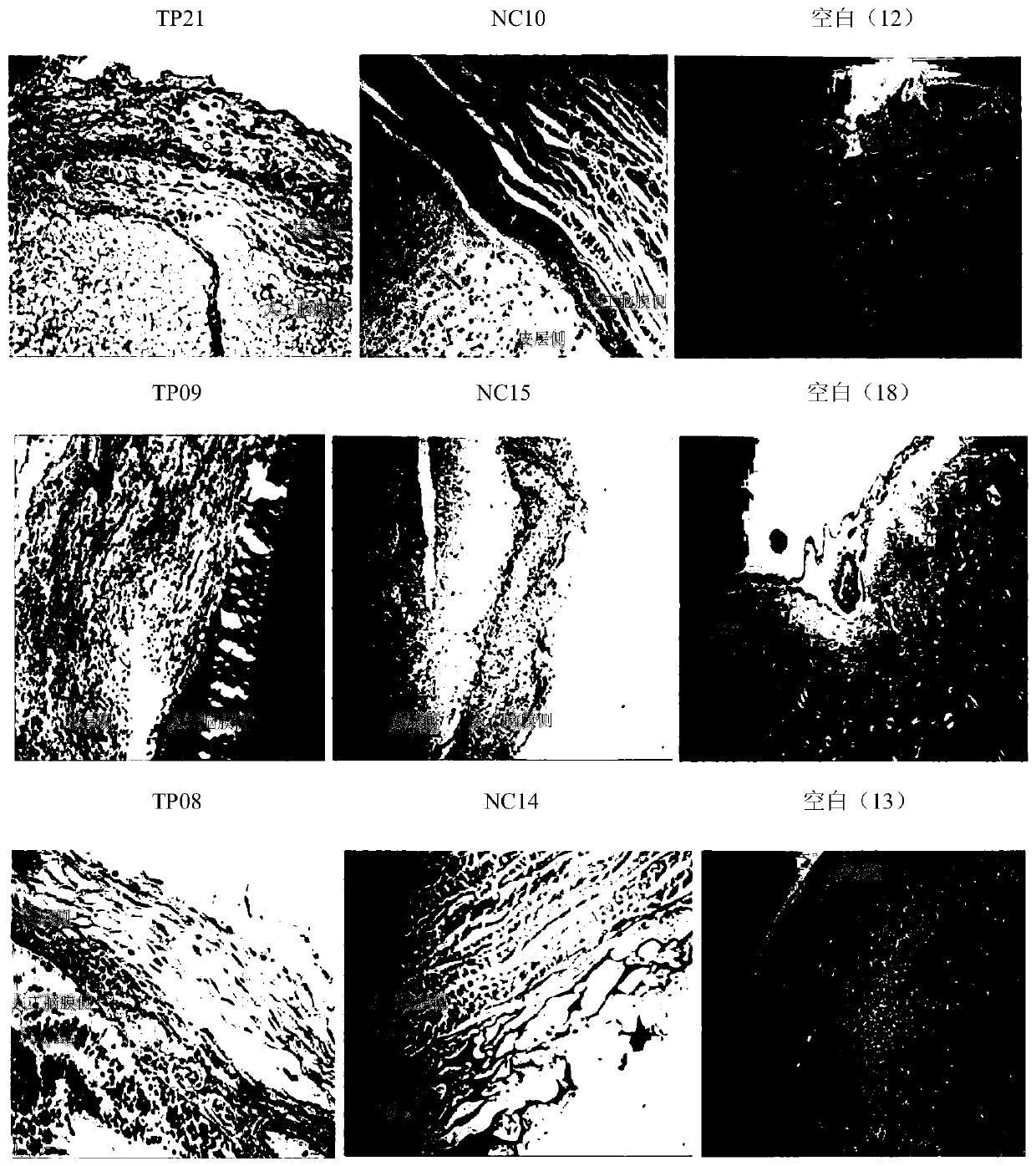
Embodiment 2
[0068] Example 2 Preparation method of acellular allogeneic dermal matrix
[0069] The present invention provides a method for preparing an acellular allogeneic dermal matrix, and a preferred preparation embodiment includes performing the following steps in order:
[0070] Raw material is put into the container that is filled with cross-linking agent solution, soaks 4 hours, and cross-linking agent is genipin and N-hydroxysuccinimide, and the mass ratio of genipin and N-hydroxysuccinimide is 4:1, the mass ratio of cross-linking agent to skin allograft is 1.4:1, soak for 8 hours. Take out the raw material, put it into another container filled with physiological saline solution, soak for 64 hours at 5°C, change the physiological saline solution every 8 hours, and obtain the semi-finished product A.
[0071] Put the semi-finished product A into 0.3% trypsin-papain solution (w / v), soak and shake, wherein, the mass ratio of trypsin and papain is 2.5:1, adjust the pH value to 7.2 w...
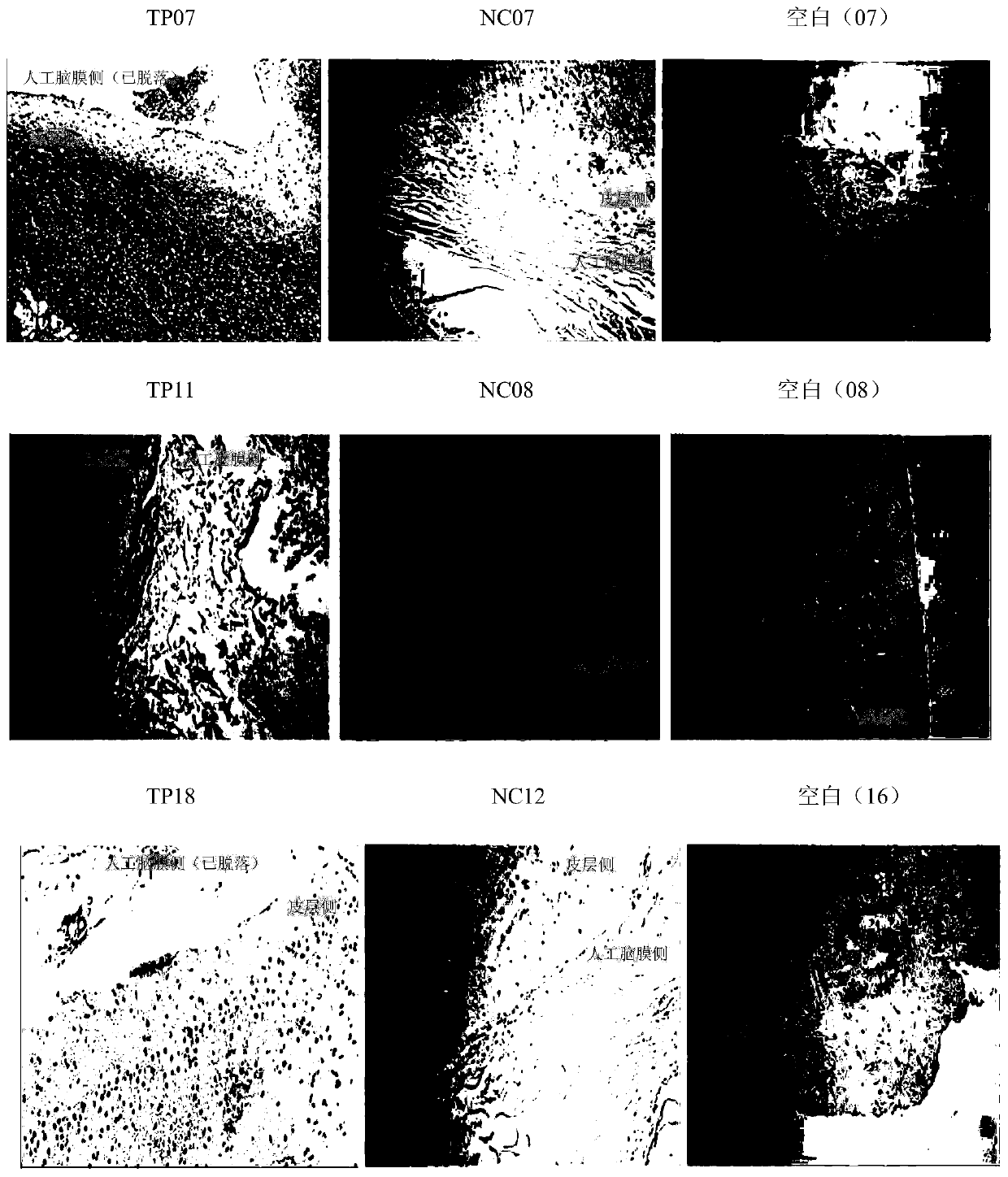
Embodiment 3
[0080] Example 3 Preparation method of acellular allogeneic dermal matrix
[0081] The present invention provides a method for preparing an acellular allogeneic dermal matrix, and a preferred preparation embodiment includes performing the following steps in order:
[0082] Raw material is put into the container that is filled with cross-linking agent solution, soaks 5 hours, and cross-linking agent is genipin and N-hydroxysuccinimide, and the mass ratio of genipin and N-hydroxysuccinimide is 3:1, the mass ratio of cross-linking agent to allograft leather is 1.5:1, soak for 8 hours. Take out the raw material, put it into another container filled with physiological saline solution, soak for 56 hours at 5°C, change the physiological saline solution every 8 hours, and obtain the semi-finished product A.
[0083] Put the semi-finished product A into 0.2% trypsin and bromelain solution (w / v) for soaking and shaking, wherein the mass ratio of trypsin and bromelain is 1:1, adjust the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bursting strength | aaaaa | aaaaa |
| suture strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com