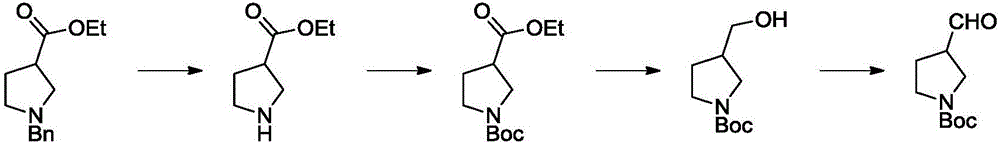
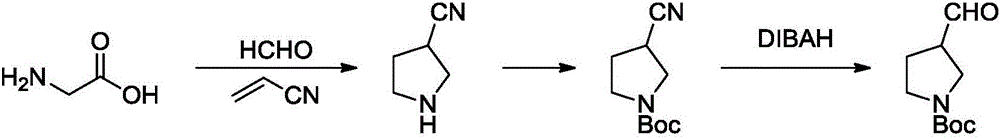
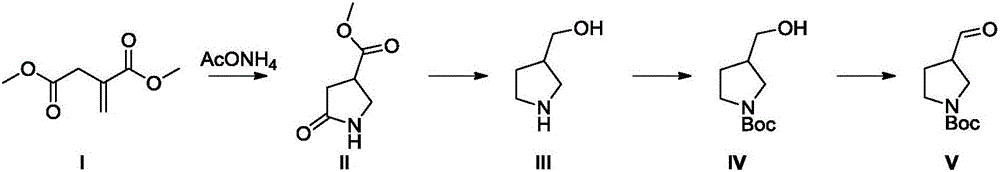
Method for synthesizing N-Boc-3-pyrrolidine formaldehyde
A technology of pyrrolidine carboxaldehyde and synthesis method, which is applied in the field of synthesis of N-Boc-3-pyrrolidine carboxaldehyde, can solve the problems of high price of Pd/C, unsuitable for scale-up production, cumbersome post-processing, etc., and achieves great practical value and Social and economic benefits, low production cost, simple and reliable method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Put 4Kg of dimethyl itaconate, 580g of camphorsulfonic acid, 50g of 4A molecular sieve and 20L of methanol into the reaction kettle, add 3.8Kg of ammonium acetate, and then raise the temperature to 50°C for 5 hours. Add water to quench the reaction, extract with ethyl acetate, wash the organic phase successively with saturated aqueous sodium bicarbonate solution and saturated brine, concentrate the organic phase to obtain a light yellow oily liquid, and obtain 3.3 Kg of methyl 5-oxo-3-pyrrolidinecarboxylate, Yield 92%.
[0034] (2) Dissolve 2 kg of methyl 5-oxo-3-pyrrolidinecarboxylate in 20 liters of tetrahydrofuran, add 1.9 kg of sodium borohydride in batches at room temperature, and slowly add 3.9 kg of boron trifluoride ether solution dropwise after the addition, After the dropwise addition, stir at room temperature for 10 minutes, heat to reflux, react for about 4 hours, add water to quench the reaction, extract with ethyl acetate, concentrate to obtain a light...
Embodiment 2
[0038] (1) Put 4Kg of dimethyl itaconate, 2.27kg of oxalic acid, 50g of 4A molecular sieve and 20L of ethanol into the reaction kettle, add 3.8Kg of ammonium acetate, and then reflux for 8 hours. The reaction was quenched by adding water, extracted with ethyl acetate, the organic phase was washed successively with saturated aqueous sodium bicarbonate solution and saturated brine, and the organic phase was concentrated to obtain 2.8 Kg of methyl 5-oxo-3-pyrrolidinecarboxylate, with a yield of 78%.
[0039] (2) Dissolve 2 kg of methyl 5-oxo-3-pyrrolidinecarboxylate in 20 liters of tetrahydrofuran, add 1.9 kg of sodium borohydride in batches at room temperature, and slowly add 3.2 kg of trifluoroacetic acid dropwise after the addition is complete. Then stir at room temperature for 10 minutes, heat to reflux, react for about 4 hours, add water to quench the reaction, extract with EA, concentrate to obtain a light yellow oily liquid, dissolve this light yellow oily liquid in 20 etha...
Embodiment 3
[0043] (1) Put 4Kg of dimethyl itaconate, 2.27kg of oxalic acid, 50g of 4A molecular sieve and 20L of ethanol into the reaction kettle, add 3.8Kg of ammonium acetate, and then reflux for 8 hours. The reaction was quenched by adding water, extracted with ethyl acetate, the organic phase was washed successively with saturated aqueous sodium bicarbonate solution and saturated brine, and the organic phase was concentrated to obtain 2.8 Kg of methyl 5-oxo-3-pyrrolidinecarboxylate, with a yield of 78%.
[0044] (2) Dissolve 2 kg of methyl 5-oxo-3-pyrrolidinecarboxylate in 20 liters of tetrahydrofuran, add 1.9 kg of sodium borohydride in batches at room temperature, and slowly add 3.9 kg of boron trifluoride ether solution dropwise after the addition, After the dropwise addition, stir at room temperature for 10 minutes, heat to reflux, react for about 4 hours, add water to quench the reaction, extract with ethyl acetate, concentrate to obtain a light yellow oily liquid, dissolve the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com