Preparation method of cyproconazole
A technology of cyproconazole and sodium methoxide, applied in the field of preparation of cyproconazole, can solve the problems of complicated process, polluted environment, unsuitable for industrial production and the like, and achieves the effects of simple raw materials and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
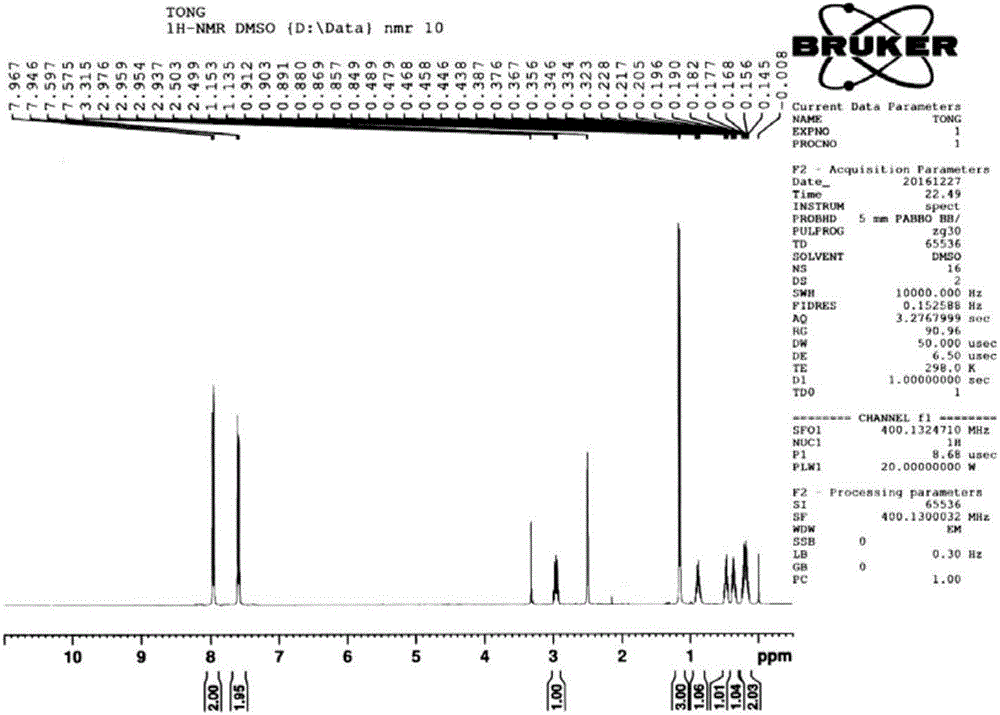
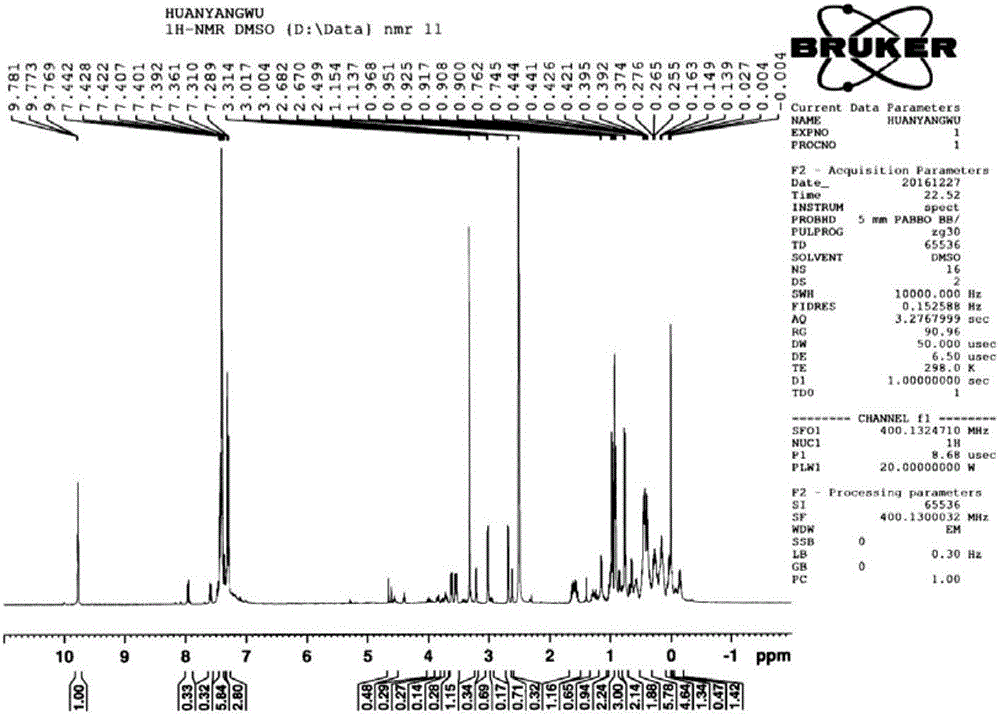
[0038] Add 100mL tetrahydrofuran and 15g magnesium chips (617mmol) into a 500ml three-necked flask equipped with a thermometer, a condenser, and a stirring device, and add 55.5g (411mmol) of bromomethylpropane and 37.7g of compound 1 (274mmol) dropwise under reflux. , 100mL tetrahydrofuran solution, add dropwise within about 1.5h, and then reflux for 2h, pour the reaction solution into ice water, acidify with 1N sulfuric acid, extract with methyl tert-butyl ether, combine the organic phases, wash with saturated brine, and dry. Methyl tert-butyl ether was distilled off under reduced pressure to obtain 52.3g of compound 2 with a yield of 98% and a purity of 98% (calculated as compound 1 p-chlorobenzonitrile). The nuclear magnetic spectrum of compound 2 is shown in Figure seven .
[0039] Add sodium methoxide (19.4g 360mmol) and 100mL DMF to a 500ml three-necked flask equipped with a thermometer, a condenser, and a stirring device. Compound 2 (38.9g 200mmol) is dissolved in 190m...
Embodiment 2
[0043] Add 60mL of n-hexane and 4.8g of magnesium chips (197mmol) into a 250ml three-necked flask equipped with a thermometer, a condenser, and a stirring device, and add 17.7g (131mmol) of bromomethylpropane, 18g of compound 1 (131mmol) dropwise under heating and reflux. ), 50mL of n-hexane solution, added dropwise within about 40min, and then refluxed for 2h, poured the reaction solution into ice water, acidified with 1N sulfuric acid, extracted with isopropyl ether, combined the organic phases, washed with saturated brine, dried, and reduced pressure The isopropyl ether was distilled off to obtain 20.4g of compound 2, with a yield of 80% (calculated as compound 1 p-chlorobenzonitrile)
[0044] Sodium methoxide (8.3g 154mmol) and 80mL N,N-dimethylacetamide were added to a 500ml three-neck flask equipped with a thermometer, condenser, and stirring device, and compound 2 (30.0g 154mmol) was dissolved in 150mL N,N-dimethylacetamide Methyl acetamide, then slowly added dropwise t...
Embodiment 3
[0048] Add 65mL of ether and 5.6g of magnesium chips (232mmol) into a 250ml three-necked flask equipped with a thermometer, a condenser, and a stirring device, and add 25.5g (189mmol) of bromomethylpropane and 20g of compound 1 (145mmol) dropwise under reflux. , 50 mL of diethyl ether solution, added dropwise within about 45 min, and then refluxed for 2 h, poured the reaction solution into ice water, acidified with 1N sulfuric acid, extracted with diethyl ether, combined the organic phases, washed with saturated brine, dried, and distilled off the diethyl ether under reduced pressure. Obtained 27.1g of compound 2 with a yield of 96% (calculated as compound 1 p-chlorobenzonitrile)
[0049] Sodium methylate (15.6g 288mmol) and 90mL dimethyl sulfoxide were added into a 500ml three-neck flask equipped with a thermometer, a condenser tube and a stirring device, and compound 2 (35.0g 180mmol) was dissolved in 150mL dimethyl sulfoxide, and then slowly Slowly added dropwise to sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com