Method for separating and purifying high-purity chicken-precursor intramuscular fat cells and establishing intramuscular-fat-cell-and-muscle-satellite-cell co-culturing system
A technique for intramuscular fat cells and muscle satellite cells, applied in the field of cytology, can solve the problems of uneven distribution of intramuscular fat tissue in chickens, inability to directly collect fat tissue, and limiting molecular regulation mechanism, etc. well-defined effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
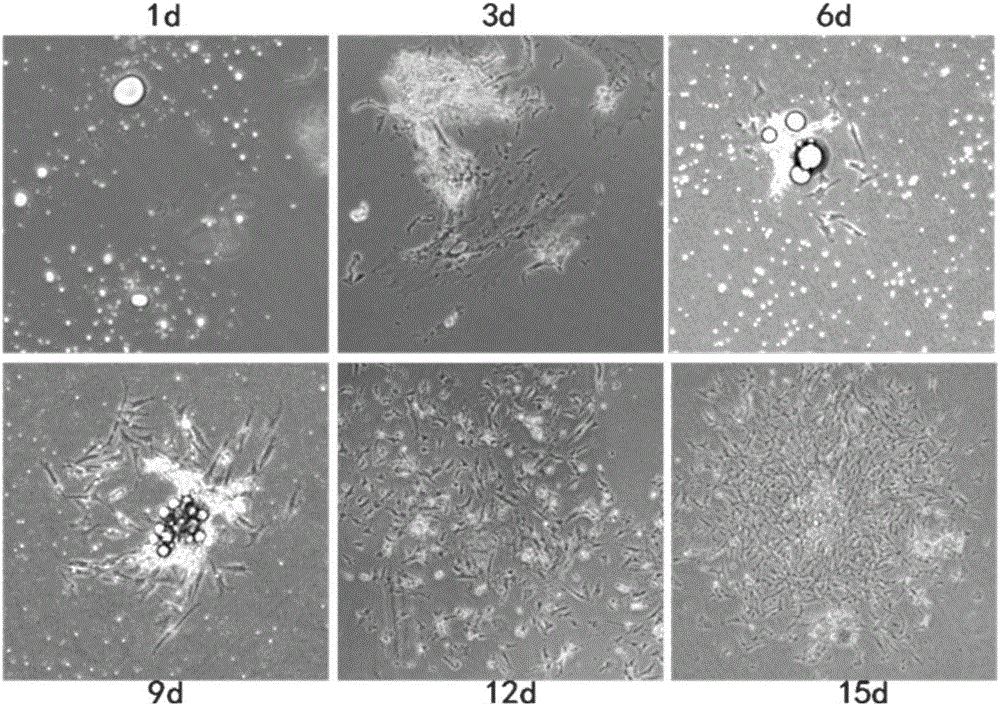
[0057] Example 1 Isolation and acquisition of high-purity chicken precursor intramuscular fat cells
[0058] 10-21-day-old Beijing oily chickens were bled to death, completely soaked in 75% (V:V) alcohol solution for 5 minutes, and separated cells on a sterile ultra-clean workbench in the cell room.
[0059] Remove the chest skin, subcutaneous fat, and connective tissue using sterile scissors and forceps. The ophthalmology department cut out the pectoralis major muscle, put it into the PBS buffer containing double antibodies (penicillin 100U / ml and streptomycin 100U / ml) and washed it 3 times, then cut the muscle tissue into 1mm 3 After the size of the minced meat, move it into a 10ml centrifuge tube, add PBS solution containing 1% double antibody and let it stand for 1 minute, wait for the muscle tissue to settle, discard the supernatant and floating tissue; then add 9 times the volume of 0.1% Type I collagenase was shaken and digested in a water bath at 37°C for 30 minutes. ...
Embodiment 2
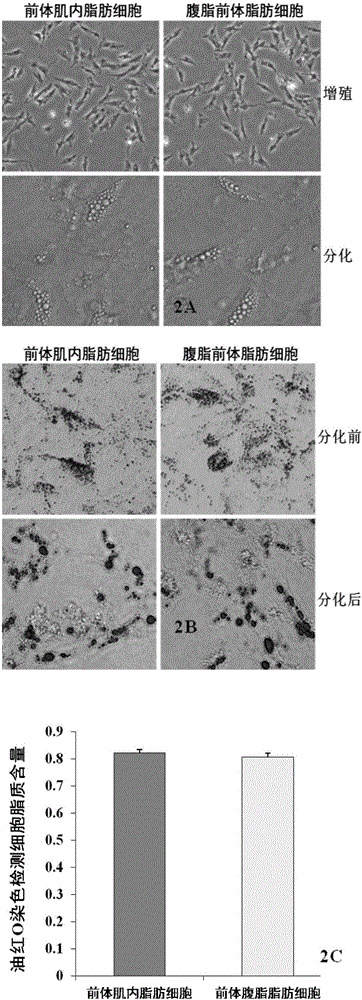
[0062] Identification of chicken precursor intramuscular fat cells obtained in Example 2
[0063] The chicken precursor intramuscular adipocytes obtained in Example 1 were separated, and the chicken abdominal fat preadipocytes obtained by existing conventional methods were used as a control, and were respectively divided into 1 × 10 5 / ml density was seeded in 6-well cell culture plates, when the cells grew to 100% confluence, the cell morphology was observed under a microscope, and the two types of cells were treated with differentiation induction and non-induction. Induced group DMEM / F12 medium (containing 10% FBS, 1% double antibody) added 10 μg / ml INS (insulin) and 1 μmol / L DEX (dexamethasone) and 115ng / ml IBMX (3-isobutyl-1 - methylxanthine) induction differentiation agent, after 96 hours of induction, all cells were tested for lipid content by Oil Red O staining, and each treatment of each cell was repeated in 3 wells. After staining, observe and take pictures under the ...
Embodiment 3
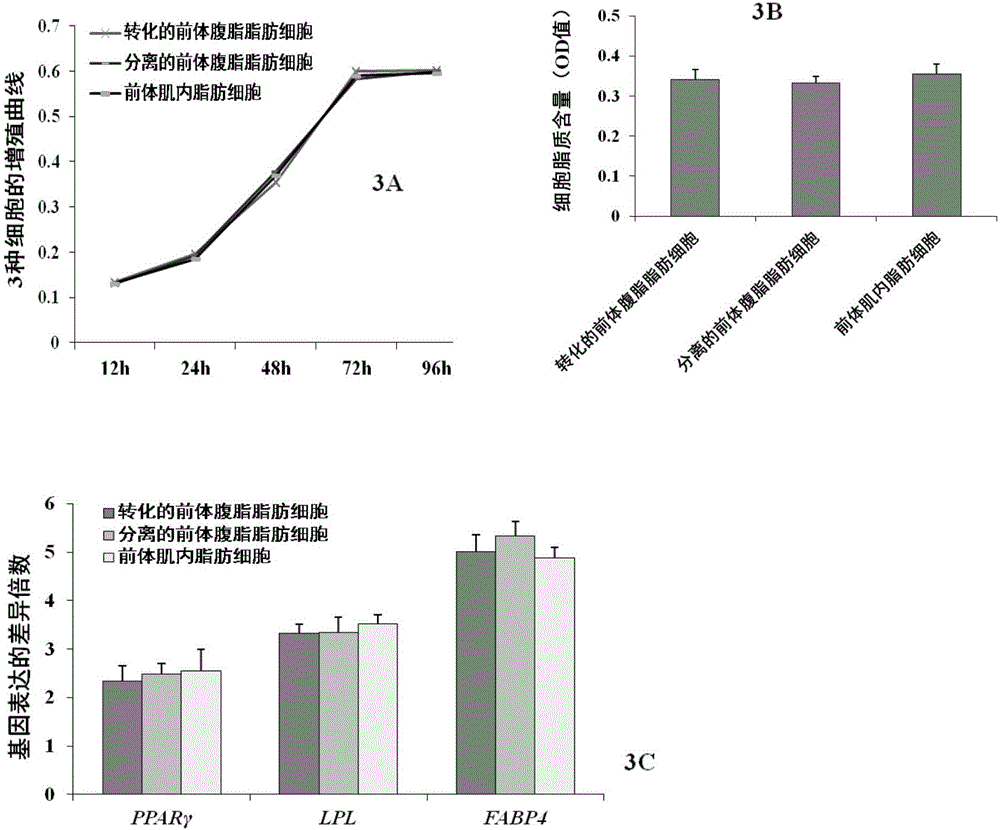
[0066] Proliferation and differentiation ability identification of chicken precursor intramuscular fat cells obtained in Example 3
[0067] The chicken pre-body intramuscular adipocytes obtained by the isolation of Example 1, the chicken abdominal fat pre-adipocytes obtained by the existing conventional method and the abdominal fat pre-adipocytes obtained by the same method as in Example 1 were used as controls, and compared The proliferation and differentiation abilities of the chicken precursor intramuscular adipocytes obtained by this method were analyzed. The three types of cells were seeded in 96-well cell culture plates at a final density of 15% of the cells, and cultured in DMEM / F12 medium (containing 10% FBS, 1% double antibody), and were cultured for 12h, 24h, 48h, 72h and 96h respectively The number of cells was detected by the MTT method, and each treatment was repeated in 8 wells. After 4 hours of MTT staining, DMSO was eluted, and the OD value of the eluate was m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com