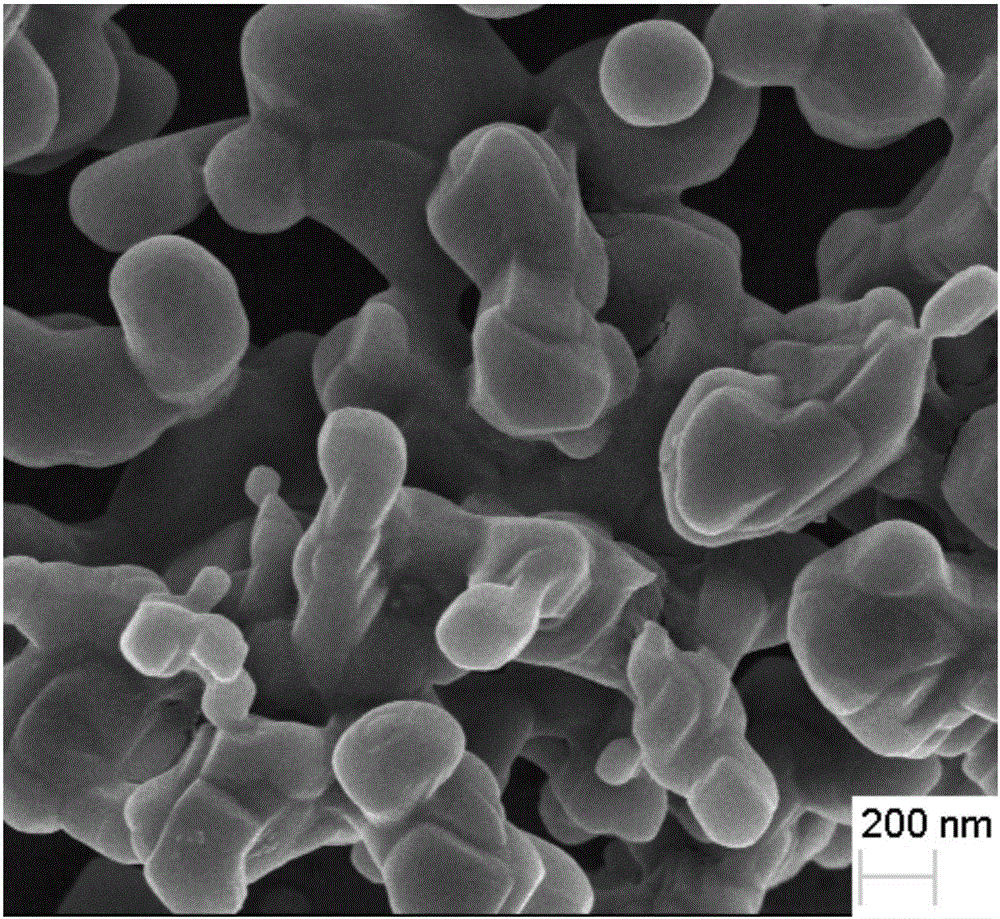
Preparation method of beta-tricalcium phosphate nanometer coating
A technology of tricalcium phosphate nanometer and tricalcium phosphate, which is applied in the direction of coating, electrolytic coating, electrophoretic plating, etc., can solve the problems of high equipment price, high requirements for preparation environment conditions, and complicated operation, so as to enhance the binding force and avoid Phase change and embrittlement, energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of the β-TCP nano coating of the present embodiment may further comprise the steps:
[0046] (1) With 0.45mol / L Ca(NO 3 ) 2 4H 2 O solution is calcium source, 0.5mol / L (NH 4 ) 2 HPO 4 The solution is a phosphorus source, adjust the amount of calcium source and phosphorus source to control the molar ratio of Ca / P to 1.5; add the dispersant polyethylene glycol to the calcium source and stir evenly; adjust the pH of the phosphorus source solution to 9.0 with ammonia water; adjust the pH The final phosphorus source solution was added dropwise to the calcium source solution under the condition of stirring at 400r / min. During the dropwise addition, the pH of the reaction solution system was kept at 7.0. After the dropwise addition, continued to stir for 10h, aged for 2d, and filtered. Wash with deionized water until the pH of the supernatant is 7.0, freeze-dry, and calcinate at 800°C for 3 hours to obtain β-TCP powder;
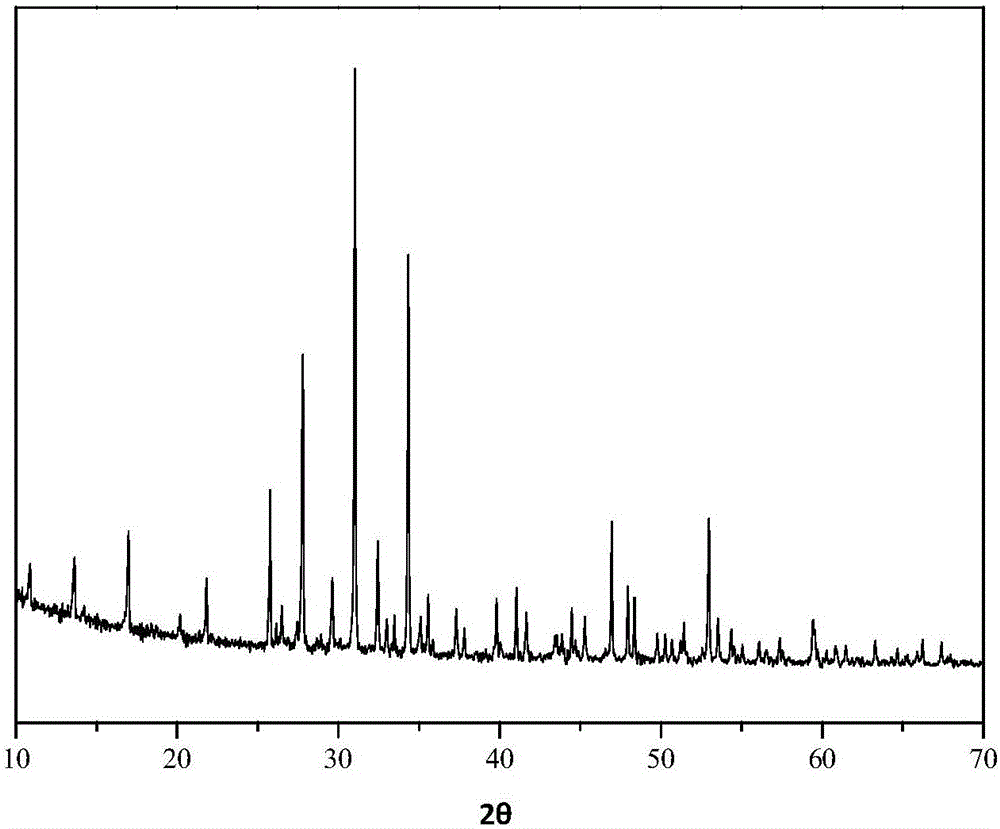
[0047] figure 1 It is the XRD pa...
Embodiment 2
[0057] The preparation of the β-TCP nano coating of the present embodiment may further comprise the steps:
[0058] (1) With 0.45mol / L Ca(NO 3 ) 2 4H 2 O solution is calcium source, 0.5mol / L (NH 4 ) 2 HPO 4 The solution is a phosphorus source, adjust the amount of calcium source and phosphorus source to control the molar ratio of Ca / P to 1.5; add the dispersant polyethylene glycol to the calcium source and stir evenly; adjust the pH of the phosphorus source solution to 9.0 with ammonia water; The solution was added dropwise to the calcium source solution under the condition of stirring at 400r / min. During the dropwise addition, the pH of the reaction solution system was kept at 7.0. After the dropwise addition, continued stirring for 10h, aging for 2d, filtering, and using Washing until the pH of the supernatant is 7.0, freeze-drying, and calcining at 800°C for 3 hours to obtain β-TCP powder;
[0059] (2) Weigh the prepared β-TCP powder and add it into ethanol to prepare...
Embodiment 3
[0068] The β-TCP nano-coating obtained by electrophoretic deposition for 5min under 25V / cm electric field strength is used for QCM-D technology, and the adsorption behavior of bovine serum albumin (BSA) on it is studied, comprising the following steps:
[0069] (1) The preparation of β-TCP nano-coating is the same as in Example 1, and the electrophoretic deposition time is 5min;
[0070] (2) Using QCM-D technology to study the adsorption behavior of bovine serum albumin (BSA) on the β-TCP nano-coating, BSA was dissolved in PBS buffer solution to prepare a 1mg / ml bovine serum albumin solution. During the experiment The PBS buffer solution was introduced first, and then the BSA solution was introduced after the baseline equilibrium was stable, and the adsorption was observed until the adsorption reached equilibrium.
[0071] Figure 9 and Figure 10 The frequency Δf and dissipation value ΔD of BSA adsorbed on the β-TCP nano-coating vary with time, respectively. It can be seen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com