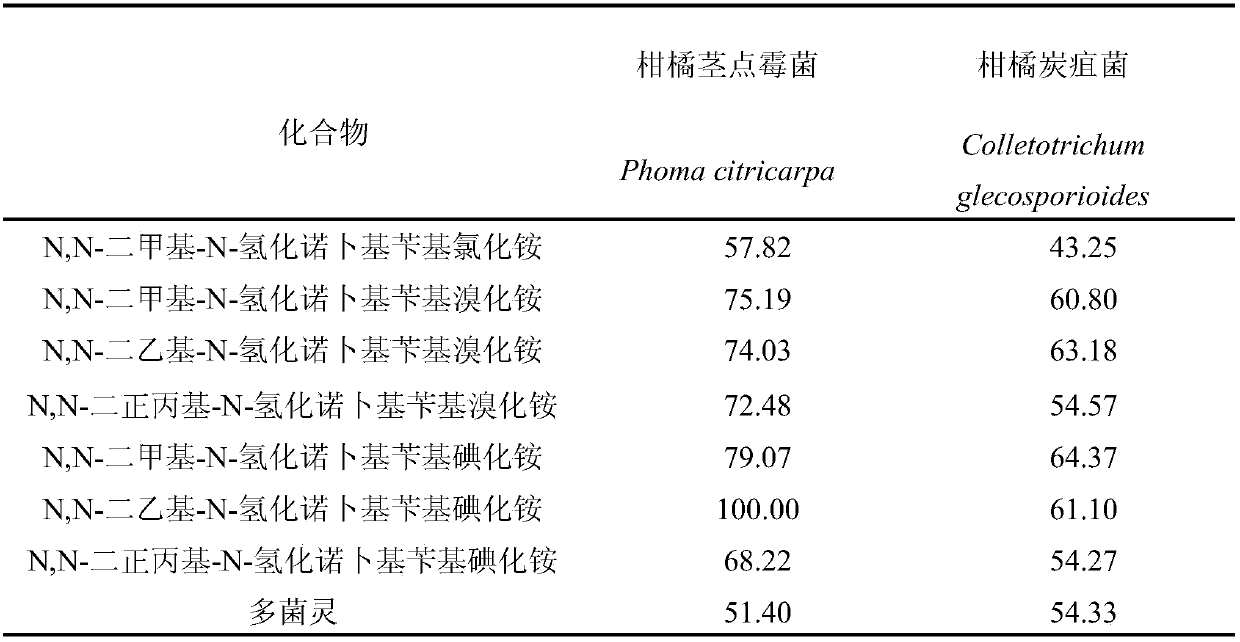
Synthetic method and antibacterial application of dialkylhydrogenated noppylbenzyl quaternary ammonium salt
A technology of dialkylhydrogenated noppylbenzyl and alkylhydrogenated noppylbenzyl, which is applied in the field of chemical synthesis of natural products, and achieves the effects of simple reaction operation, good inhibition effect and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
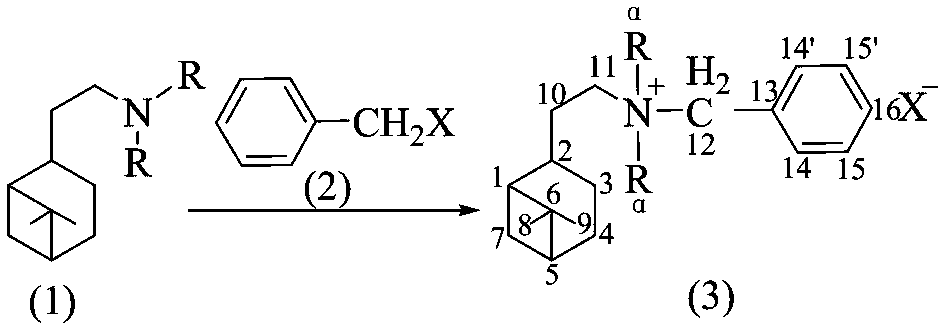
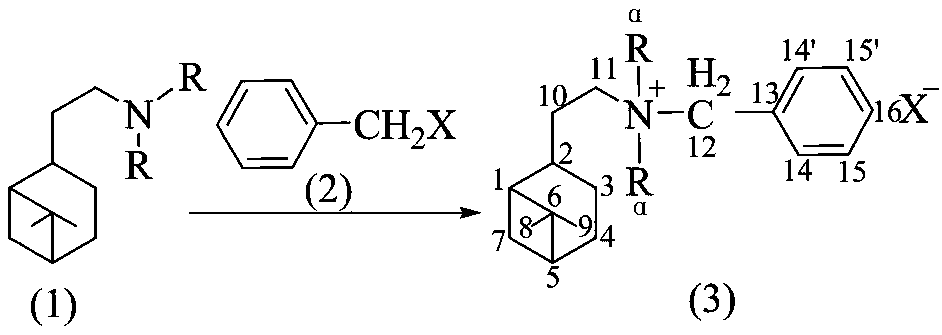
[0021] General Synthesis of Dialkyl Hydrogenated Noppylbenzyl Bromide (Chlorine)
[0022] Add about 0.02mol of dialkylhydrogenated nopylamine, 0.025mol of benzyl halide (bromine, chlorine) and appropriate amount of toluene into a 100mL Erlenmeyer flask equipped with a stirring bar and a condensing reflux device, and heat at 100-150°C 1-3d, take out the product, cool and crystallize, wash the obtained crystal with cold petroleum ether (60-90°C), filter with suction, wash with petroleum ether several times and then pump to dryness, then depressurize to remove possible residual solvent and dried to obtain the product.
Embodiment 2
[0024] The benzyl halide is benzyl chloride, the tertiary amine is N,N-dimethylhydrogenated noppylamine, and other experimental methods and conditions are the same as in Example 1 to obtain N,N-dimethyl-N-hydrogenated noppylamine Benzyl ammonium chloride.
[0025] MS (C 20 h 32 NCl): 356.4 (M + +Cl), 286.4 (M + -Cl), 677.3 (2M + +Cl), 607.4 (2M + -Cl),35(Cl);
[0026] 1 H NMR,δ H / ppm:7.686(2H,d,J=6.8Hz, 14- CH, 14’- CH),7.442(3H,t,J 1 =J 2 =6.8Hz, 15- CH, 15’- CH, 16- CH),5.084(2H,s, 12- CH),3.536(2H,m, 11- CH 2 ),2.311(1H,m, 2- CH),2.017~1.829(8H,m, 7- CH, 10- CH 2,3- CH, 5- CH, 1- CH, 4- CH 2 ),1.456(1H,m, 3- CH),1.196(3H,s, 9- CH 3 ),1.034(3H,s, 8- CH 3 ),0.825(1H,d,J=9.2Hz, 7- CH);
[0027] 13 C NMR,δ C / ppm:38.537(C -1 ), 46.113 (C -2 ), 22.089 (C -3 ),26.060(C -4 ), 41.040 (C -5 ), 38.537 (C -6 ), 30.182 (C -7 ), 23.313 (C -8 ), 27.923 (C -9 ), 33.360 (C -10 ), 67.242 (C -11 ), 62.797 (C -12 ), 127.497 (C -13 ), 133.211 (C ...
Embodiment 3
[0030] The benzyl halide is benzyl bromide, the tertiary amine is N,N-dimethylhydrogenated noppylamine, and other experimental methods and conditions are the same as in Example 1 to obtain N,N-dimethyl-N-hydrogenated noppylamine Benzyl ammonium bromide.
[0031] MS (C 20 h 32 NBr):446.2(M + +Br),286.4(M + -Br), 811.2 (2M + +Br),653.3(2M + -Br), 80.2(Br);
[0032] 1 H NMR,δ H / ppm:7.702(2H,d,J=6.8Hz, 14- CH, 14’- CH),7.441(3H,s, 15- CH, 15’- CH, 16- CH),5.098(2H,s, 12- CH 2 ),3.601(2H,m, 11- CH 2 ),3.296(6H,s,2 α- CH 3 ),2.305(1H,m, 2- CH),2.017~1.714(8H,m, 7- CH, 10 -CH 2,3- CH, 5- CH, 1- CH, 4- CH 2 ),1.470(1H,m, 3- CH),1.193(3H,s, 9- CH 3 ),1.034(3H,s, 8- CH 3 ),0.815(1H,d,J=9.2Hz, 7- CH);
[0033] 13 C NMR,δ C / ppm:38.501(C -1 ), 46.068 (C -2 ),22.100(C -3 ), 26.066 (C -4 ), 41.026 (C -5 ), 38.501 (C -6 ),30.166(C -7 ), 23.338 (C -8 ), 27.913 (C -9 ), 33.332 (C -10 ), 67.165 (C -11 ), 62.952 (C -12 ), 127.358 (C -13 ), 133.211 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com