Preparation method of (Z)-2-(tert-methoxycarbonyl methoxyimino)-2-(2-aminothiazol-4-yl)acetic acid
A technology of cefixime side chain acid and cefixime ring-opening side chain acid, applied in the direction of organic chemistry, etc., can solve problems such as hidden dangers of production safety, increase process flow, increase production cost, etc. The effect of simple handling and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0021] This embodiment relates to a method for preparing cefixime side chain acid, including the following steps:
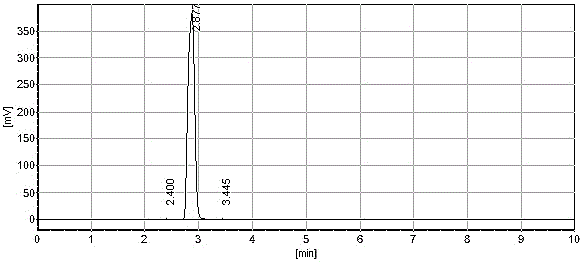
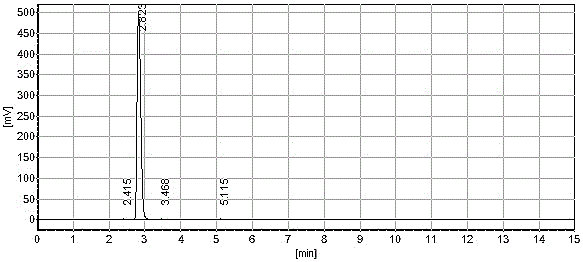
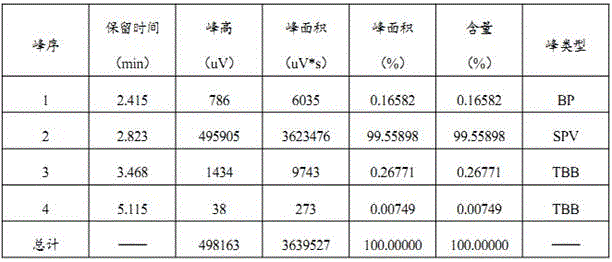
[0022] Cefixime ring-opening side chain acid is prepared by laboratory synthesis reaction. Using tert-butyl acetoacetate as raw material, cefixime ring-opening side chain acid is synthesized through oximation, alkylation, and chloro-acid hydrolysis. The purity is greater than 92. %;
[0023] a. In a 500ml four-necked bottle, add 150g water and 100g cefixime ring-opening side chain acid successively, control the temperature at 15~20℃, and slowly add ammonium bicarbonate until the cefixime ring-opening side chain acid is dissolved, and then use Adjust the pH value of ammonium bicarbonate to 4.5±0.2, then add activated carbon for decolorization, stir at 15°C for 30 minutes, and filter to obtain the ammonium salt aqueous solution of cefixime ring-opening side chain acid; the amount of ammonium bicarbonate added in this step is 30.67g;
[0024] b. Add 34.6g of thiourea, 230...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com