(S)-pantoprazole preparation method
A technology for pantoprazole and pantoprazole sulfide, which is applied in the field of drug synthesis, can solve the problems of low enantioselectivity, complicated post-processing, expensive titanium reagents and the like, and achieves good selectivity, simple post-processing, The effect of shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
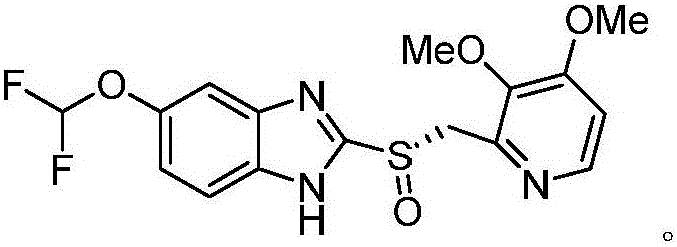
[0029] Preparation of (S)-pantoprazole
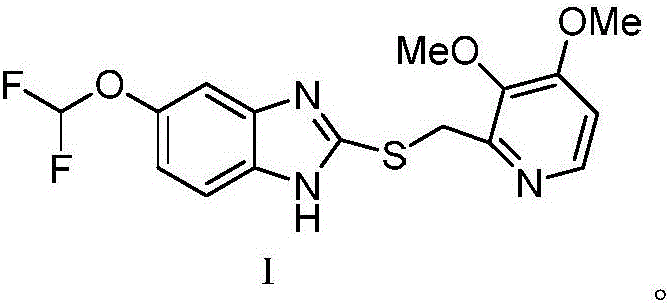
[0030] Under nitrogen protection, at room temperature, pantoprazole sulfide 36.74g (100mmol) and chiral quaternary ammonium salt 19.78g (quinine benzyl quaternary ammonium salt-bromine, 40mmol) were added in 150ml acetonitrile and stirred for premixing for 30min, then added N-Methyl-N-morpholine oxide 23.43g (200mmol), then heated to 50 ° C, continued to stir the contact reaction for 1.5 hours, the reaction solution was filtered, the filtrate was concentrated, washed with water, then recrystallized from petroleum ether, and dried to obtain (S)- Pantoprazole is 37.7g, the yield is 98.3%, the ee value is 99.63%, and no over-oxidized sulfone by-product is detected.
Embodiment 2
[0032] Preparation of (S)-pantoprazole
[0033] Under nitrogen protection, at room temperature, pantoprazole sulfide 36.74g (100mmol) and chiral quaternary ammonium salt 9.89g (quinine benzyl quaternary ammonium salt-bromine, 20mmol) were added in 180ml acetonitrile and stirred and premixed for 30min, then added N-methyl-N-morpholine oxide 17.57g (150mmol), then heated up to 45°C, continued stirring and contact reaction for 1 hour, filtered the reaction solution, concentrated the filtrate, washed with water, then recrystallized petroleum ether, and dried to obtain (S)- Pantoprazole is 37.8g, the yield is 98.7%, the ee value is 99.51%, and no over-oxidized sulfone by-product is detected.
Embodiment 3
[0035] Preparation of (S)-pantoprazole
[0036] Under nitrogen protection, at room temperature, pantoprazole sulfide 36.74g (100mmol) and chiral quaternary ammonium salt 24.72g (quinine benzyl quaternary ammonium salt-bromine, 50mmol) were added in 180ml acetonitrile and stirred for pre-mixing for 30min, then added N-methyl-N-morpholine oxide 17.57g (150mmol), then heated up to 40°C, continued stirring and contact reaction for 1.5 hours, filtered the reaction solution, concentrated the filtrate, washed with water, then recrystallized petroleum ether, and dried to obtain (S)- Pantoprazole 37.5g, the yield is 97.7%, the ee value is 99.39%, no excessive oxidation of sulfone by-products is detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com