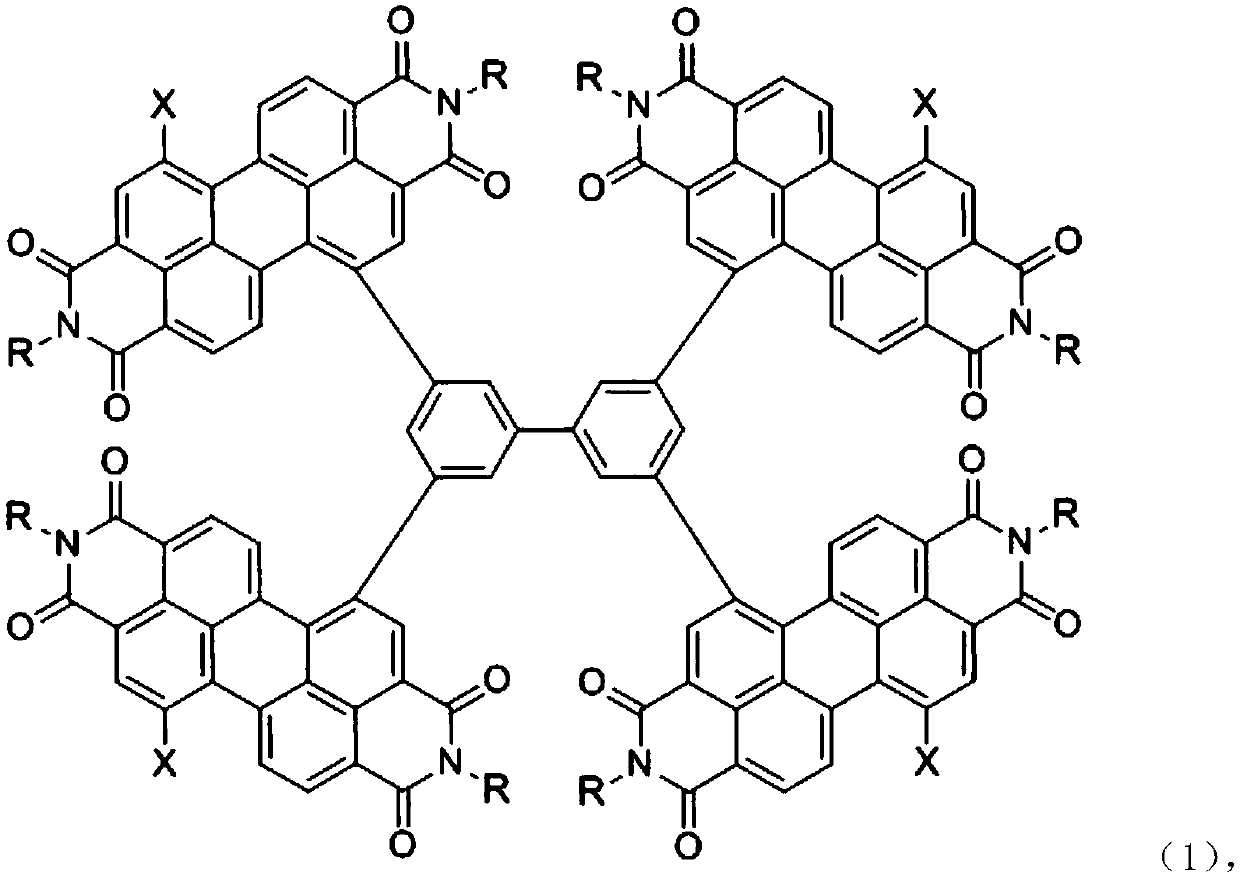
A kind of perylene imide compound and its preparation method and application
A technology of perylene imide and compound, applied in the field of polymer synthesis, can solve problems such as large charge loss, and achieve the effects of wide absorption, improved electron mobility, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
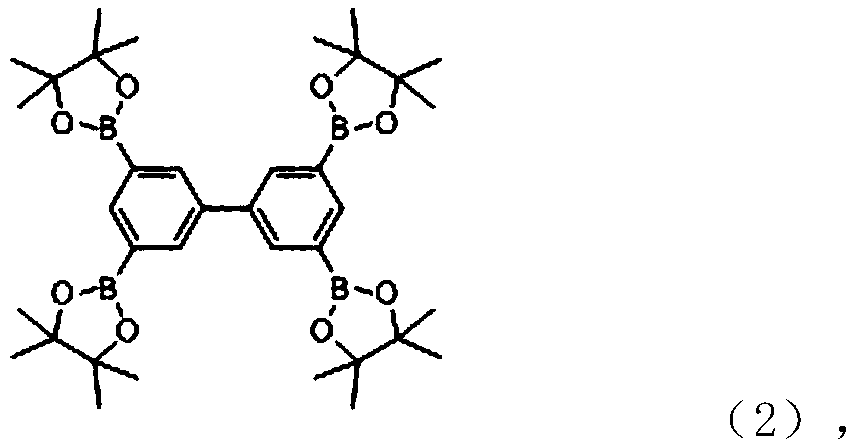
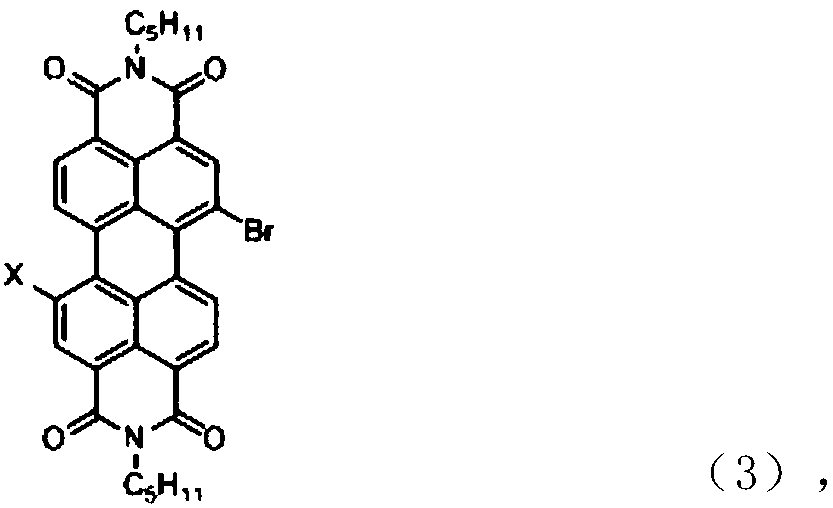
[0025] Take 3,3',5,5'-biphenyltetraboronic acid pinacol ester (1.52mmol, 1g), N,N'-bis(2-ethylpropyl)-1-bromo-perylenediimide Amine (8eq, 12.16mmol, 7.41g), 200mg Pd(PPh 3 ) 4 、K 2 CO 3 The mixed solution (containing 200mL toluene, 8mL ethanol, where K 2 CO 3 Concentration is 2mol / L) in a 500mL flask, after passing nitrogen, raise the temperature to 80°C, react for 48h, then dilute the reaction solution with dichloromethane and wash with saturated brine, collect the organic layer and dry it, then remove the dichloromethane by rotary evaporation , and then carried out column chromatography with petroleum ether / dichloromethane as the eluent to obtain 1.9 g of the target product (molecular formula shown in 3a below), with a yield of 54%.
[0026]
Embodiment 2
[0028] Take 3,3',5,5'-biphenyltetraboronic acid pinacol ester (1.52mmol, 1g), N,N'-diisooctyl-1-bromo-7-perylenediimide (8eq , 12.16mmol, 8.43g), 200mg Pd(PPh 3 ) 4 and K 2 CO 3 The mixed solution (containing 200mL toluene, 8mL ethanol, where K 2 CO 3 The concentration is 2mol / L) was placed in a 500mL flask, and after nitrogen was passed through, the temperature was raised to 80°C, and the reaction was carried out for 48h. Then dilute the reaction solution with dichloromethane and wash with saturated brine, collect the organic layer and dry it, then remove the dichloromethane by rotary evaporation and use petroleum ether / dichloromethane as eluent for column chromatography to obtain the compound 3b with the following structural formula ( 2.5 g, 63.2%).
[0029]
Embodiment 3
[0031] Take 3,3',5,5'-biphenyltetraboronic acid pinacol ester (1.52mmol, 1g), N,N'-di(2-pentylhexyl)-1-bromo-perylenediimide (8eq, 12.16mmol, 9.46g), 200mg Pd(PPh 3 ) 4 and K 2 CO 3 The mixed solution (containing 200mL toluene, 8mL ethanol, where K 2 CO 3 The concentration is 2mol / L) was placed in a 500mL flask, and after nitrogen was passed through, the temperature was raised to 80°C, and the reaction was carried out for 48h. Then dilute the reaction solution with dichloromethane and wash with saturated brine, collect the organic layer and dry it, then remove the dichloromethane by rotary evaporation and use petroleum ether / dichloromethane as eluent for column chromatography to obtain the compound 3c (3.09g , 73.2%).
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com