Sterically-hindered pyrimidine iridium complex phosphorescence material and preparation method thereof
A technology of phosphorescent materials and iridium complexes, which is applied in the field of sterically hindered pyrimidine iridium complex phosphorescent materials and its preparation, can solve the problems of low purity of blue light, improve luminous efficiency, reduce red shift, increase sublimation performance and The effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
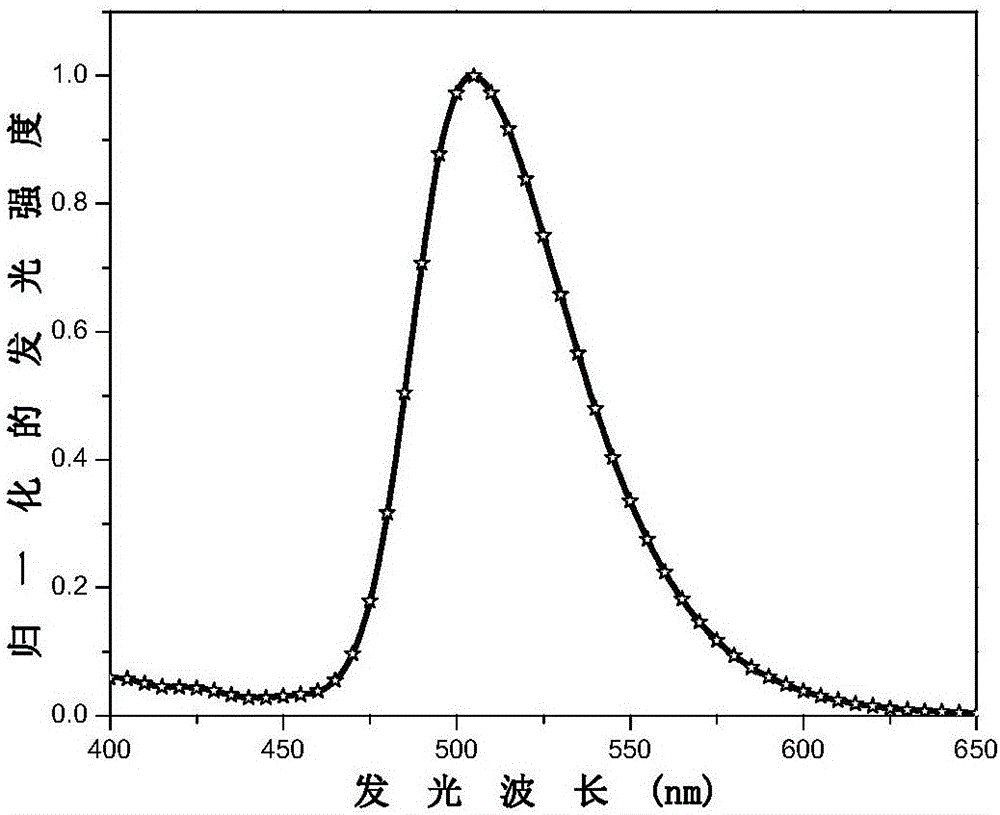
[0079] This embodiment prepares the electrophosphorescent material with the following structure:
[0080]
[0081] Bis(4-m-trimethylphenyl-6-phenylpyrimidine-N,C 2’ )(3-trifluoromethyl-5-(2'-pyridyl)-1,2,4-triazole) iridium.
[0082] A, 4-chloro-6-phenylpyrimidine synthesis;
[0083]
[0084] Weigh 2',3',4'-trisubstituted phenylboronic acid 2g (0.016mol), 4,6-dichloropyrimidine 2.45g (0.016mol), tetrakis(triphenylphosphine)palladium 0.2g and anhydrous carbonic acid Sodium 3.0g was placed in a 120mL sealed tube, and a mixed solution of 20mL tetrahydrofuran and 20mL deionized water was used as a solvent to place in the sealed tube, a stirring bar was added, and the bottle was capped. After the whole device was evacuated and replaced by nitrogen for 3 to 4 times, it was placed in an oil bath, stirred and heated on a magnetic heating stirrer to 120°C for about 8 hours. After the reaction, the closed tube was cooled to room temperature, the solution in the sealed tube was ...
Embodiment 2
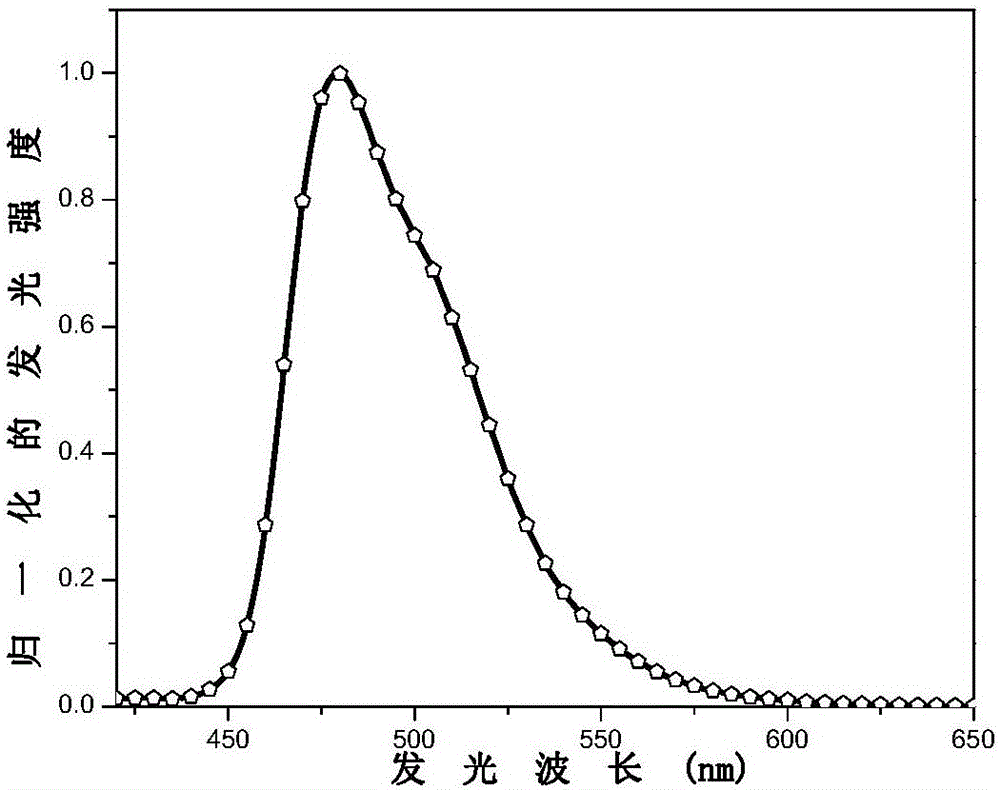
[0106] This embodiment prepares the electrophosphorescent material with the following structure:
[0107]
[0108] Bis(4-m-trimethylphenyl-6-(4’-fluorophenyl)pyrimidine-N,C 5’ )(3-trifluoromethyl-5-(2'-pyridyl)-1,2,4-triazole) iridium.
[0109] Synthesis of A, 4-chloro-6-(4'-fluorophenyl) pyrimidine;
[0110]
[0111] Weigh 2g (0.014mol) of 4-fluorophenylboronic acid, 2.14g (0.0142mol) of 4,6-dichloropyrimidine, Pd(dppf)Cl 2 0.15 g and 2.5 g of anhydrous potassium carbonate were placed in a 120 mL sealed tube, and a mixed solution of 60 mL of dioxane and 20 mL of deionized water was used as a solvent to place in the sealed tube, a stirring bar was added, and the bottle was capped. After the whole device was evacuated and replaced by nitrogen for 3 to 4 times, it was placed in an oil bath, stirred and heated on a magnetic heating stirrer to 120°C for about 8 hours. After the reaction, the closed tube was cooled to room temperature, the solution in the sealed tube was p...
Embodiment 3
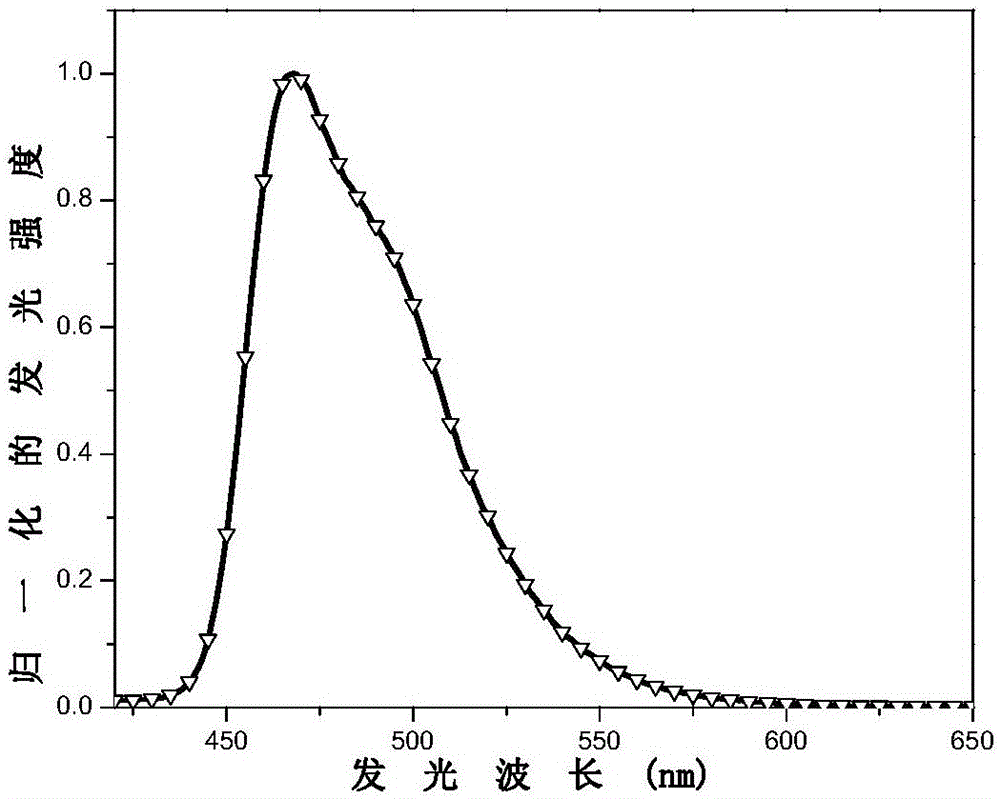
[0135] This embodiment prepares the electrophosphorescent material with the following structure:
[0136]
[0137] Bis(4-m-trimethylphenyl-6-(2’,4’-difluoro-phenyl)pyrimidine-N,C 5’ )(3-trifluoromethyl-5-(2'-pyridyl)-1,2,4-triazole) iridium.
[0138] Synthesis of A, 4-chloro-6-(2',4'-difluorophenyl)pyrimidine;
[0139]
[0140] Weigh 1.58g (0.1mol) of 2,4-difluorophenylboronic acid, 1.50g (slight excess of 0.1mol) of 4,6-dichloropyrimidine, 0.1g of tetrakis(triphenylphosphine)palladium and 2g of anhydrous sodium carbonate In a 120mL sealed tube, place a mixed solution of 30mL tetrahydrofuran and 20mL deionized water as a solvent in the sealed tube, add a stirring bar, and cap the bottle. After the whole device was evacuated and replaced by nitrogen for 3 to 4 times, it was placed in an oil bath, stirred and heated on a magnetic heating stirrer to 120°C for about 8 hours. After the reaction, the closed tube was cooled to room temperature, the solution in the sealed tub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com