A method of preparing fluororubber through microemulsion polymerization
A technology of microemulsion polymerization and fluororubber, which is applied in the field of preparation of fluororubber by microemulsion polymerization, can solve problems such as application research blanks, and achieve the effects of avoiding gel, improving vulcanization, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In this example, fluoroether carboxylate and fluoroether oil are used as raw materials to prepare microemulsion surfactants:
[0025] Add 4kg of deionized water to a 10L container, and 1.8kg of structural formula is CF 3 O(CF(CF 3 ) CF 2 O) 3 (CF 2 CF 2 O) 5 COONH 4The fluoroether carboxylate, 1.0kg structural formula is CF 3 O(CF(CF 3 ) CF 2 O) 12 (CF 2 CF 2 O) 2 CF 3 The fluoroether oil is stirred uniformly under normal temperature and pressure to obtain an aqueous solution of a microemulsion surfactant with a concentration of 41.2%.
Embodiment 2
[0027] Add 4kg of deionized water to a 10L container, and 3.8kg of structural formula is CF 3 O(CF(CF 3 ) CF 2 O) 8 COONH 4 The fluoroether carboxylate, 1.6kg structural formula is CF 3 O(CF(CF 3 ) CF 2 O) 10 (CF 2 CF 2 O) 4 CF 3 The fluoroether oil is stirred uniformly under normal temperature and pressure to obtain a concentration of 57.4% in the aqueous solution of the microemulsion surfactant.
Embodiment 3
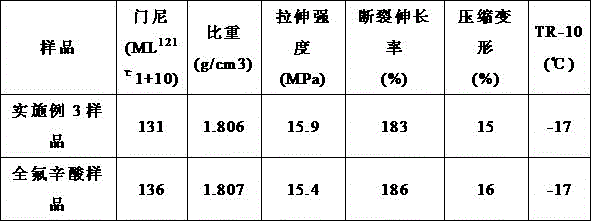
[0029] Taking a 100L polymerization kettle as an example, add 60kg of ion-free water and 1.2kg of the microemulsion surfactant prepared in Example 1 into the reaction kettle; after nitrogen replacement and vacuum treatment, the oxygen content in the kettle is ≤ 20ppm; Add vinylidene fluoride and hexafluoropropylene mixed monomers with a mass ratio of 60:40, until the pressure of the kettle is 2.1 Mpa; stir, heat up to 92°C, add 1.2Kg of potassium persulfate initiator with a concentration of 5% to start the reaction, and the reaction During the process, the monomer mixture is continuously added to keep the pressure at 2.1Mpa; when a certain amount of reaction is stopped, the reaction is stopped, and the unreacted mixed monomer is recovered after cooling; Obtain binary fluorine rubber raw rubber.
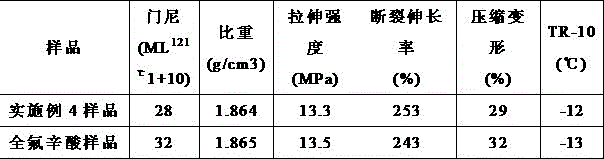
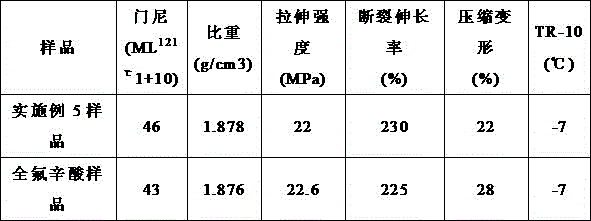
[0030] See Table 1. The fluororubber prepared in Example 3 is compared with the fluororubber prepared by using perfluorooctanoic acid as an emulsifier, and the rest of the operations ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com