Dimethyl sulfonate of compound A, crystal form of dimethyl sulfonate, and medicinal composition containing dimethyl sulfonate
A kind of technology of dimethanesulfonic acid salt and compound, applied in the field of pharmaceutical composition containing this salt, can solve problems such as unknown compound A
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation of embodiment 1 compound A
[0071] According to the method of Example 28 and 28A of the PCT / GB2012 / 051783 instruction manual, the following technical synthesis route was used to prepare compound A-methanesulfonate and its crystal form B:
[0072]
[0073] The reaction conditions and parameters are:
[0074] to N at 0°C 1 -(2-Dimethylaminoethyl)-5-methoxy-N 1 -Methyl-N 4 -[4-(1-Methylindol-3-yl)pyrimidin-2-yl]benzene-1,2,4-triamine (Intermediate 100, 10 g, 21.32 mmol) in THF (95 mL) and water ( To the stirred solution in 9.5 mL) was added 3-chloropropionyl chloride (3.28 g, 25.59 mmol). The mixture was stirred at room temperature for 15 minutes, then NaOH (3.48 g, 85.28 mmol) was added. The resulting mixture was heated to 65°C for 10 hours. The mixture was then cooled to room temperature and CH was added 3 OH (40 mL) and water (70 mL). The resulting mixture was stirred overnight. The resulting solid was collected by filtration, washed with wa...
Embodiment 2
[0079] The preparation of embodiment 2 compound A dimesylate
[0080] Add 12g of compound A-methanesulfonate to 125ml of ethanol and 25ml of water, stir at 60°C for 0.75h, dissolve, add 5.5g of methanesulfonic acid dropwise, keep stirring for 5h, then move to 20°C and stir for 14h , a pale yellow solid was precipitated, and dried at 42.5°C for 3.5 hours to obtain compound A dimesylate.
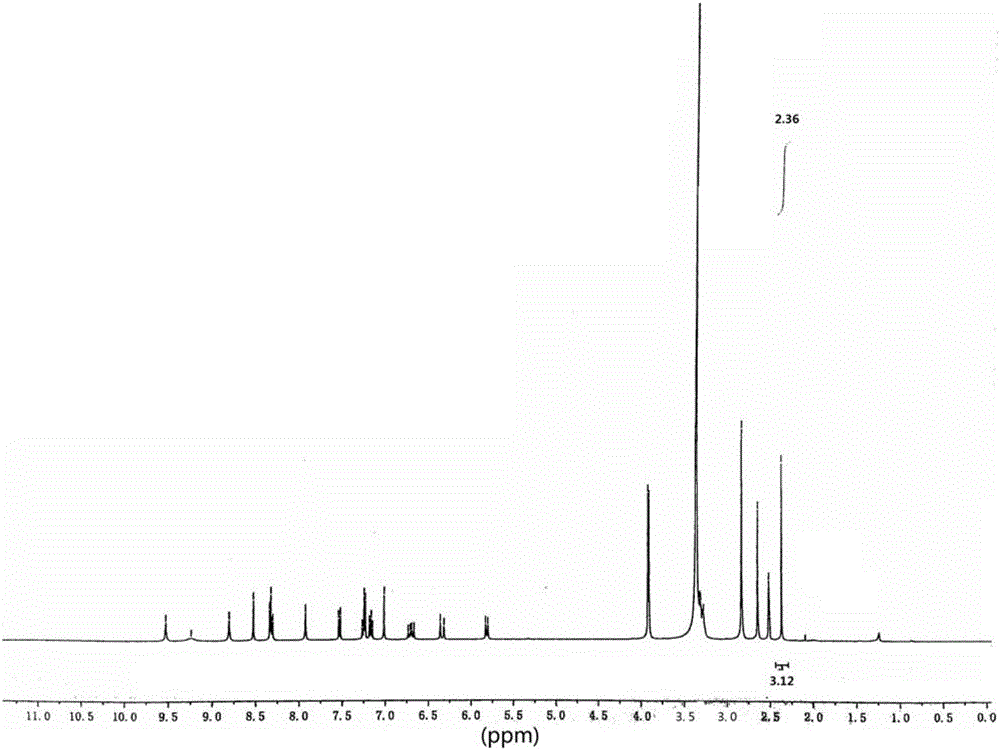
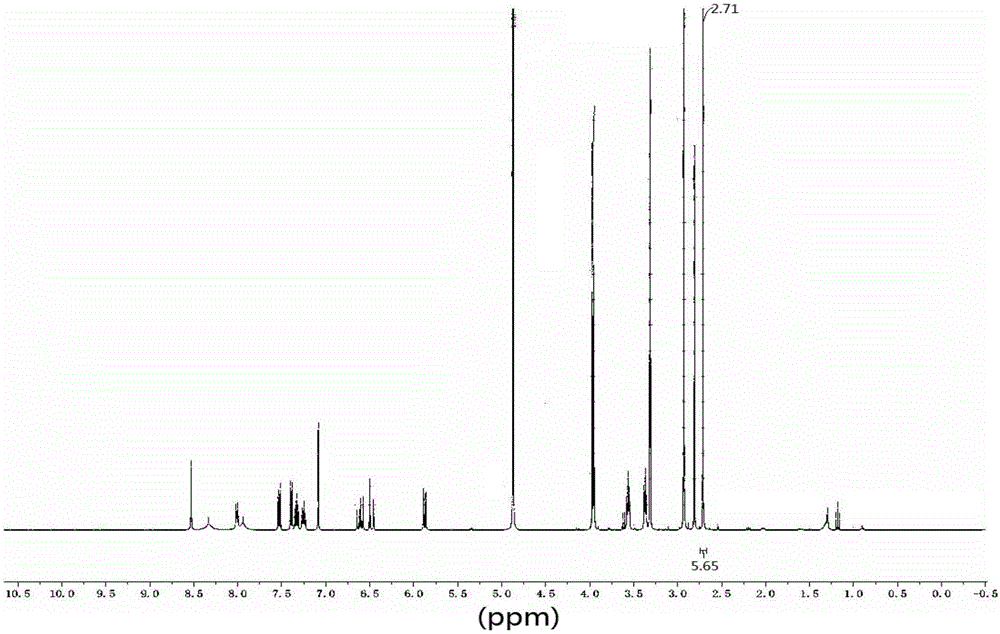
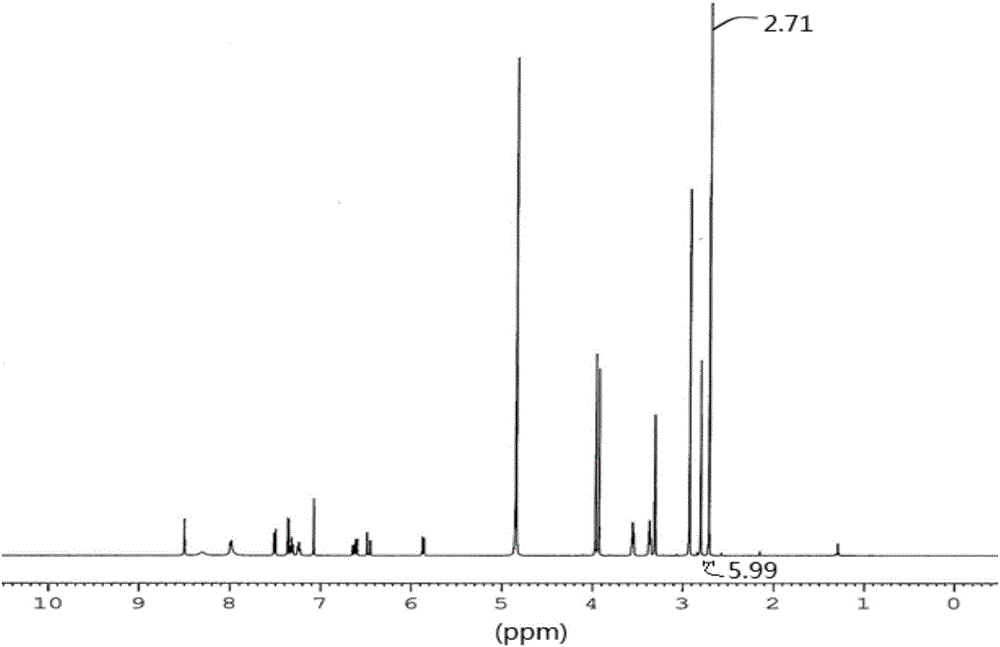
[0081] Described compound is determined by NMR, 1 H-NMR test spectrum such as figure 2 As shown in the figure, it is found that the dimethyl proton signal peak of dimethanesulfonic acid appears in the high field, indicating that there are 6 hydrogens, indicating that the obtained product is a double salt, namely dimethanesulfonate.
[0082] 1 H-NMR testing equipment and conditions: testing equipment: Bruker AVANCE III HD 500 superconducting pulse Fourier transform nuclear magnetic resonance spectrometer; testing conditions: solvent: MeOD-d4; temperature: 25°C; testing basis: JY / T 007- 1996...
Embodiment 3
[0083] Example 3 Preparation of compound A dimesylate salt crystal form α
[0084] Add 10 g of compound A dimesylate of Example 2 into 100 ml of acetonitrile, add 30 ml of water at room temperature, stir and react at 60°C for 0.5 h, and dissolve; add 100 g of acetonitrile at 30°C, stir for 1.5 h, and then Move it to an ice bath and stir for another 2 hours, a light yellow solid precipitates out, and dry at 50°C for 5 hours to obtain the product, which is the dimesylate α crystal form.
[0085] The solid material is defined as dimesylate salt α crystal form.
[0086] The X-ray diffraction pattern of obtained compound A dimesylate salt crystal form α is as follows: Figure 4 shown. The specific characteristic absorption peaks are shown in Table 1, with an error of ±0.2°.
[0087] The DSC spectrum of obtained compound A dimesylate salt crystal form α is as follows Figure 5 As shown, there is a maximum absorption peak at 262.1°C±2°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com