Immediate release cinacalcet hydrochloride preparation and preparation method thereof
A technology of cinacalcet hydrochloride instant and cinacalcet hydrochloride, which is applied in the field of cinacalcet hydrochloride immediate-release preparations and its preparation, can solve the limitation of the applicable population and the poor dissolution rate of cinacalcet hydrochloride instant preparations and other issues to achieve the effect of expanding the population of drug users
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0023] The immediate-release formulation of cinacalcet hydrochloride in this embodiment is a 25 mg cinacalcet tablet.
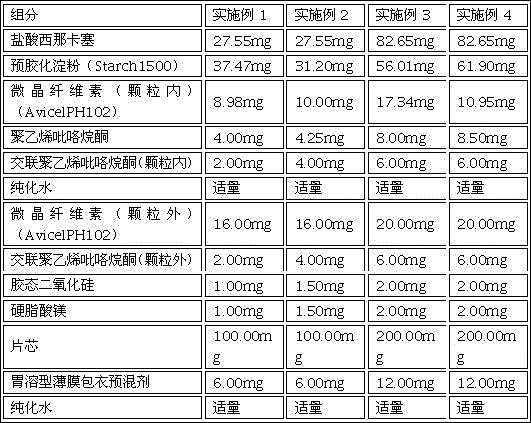
[0024] The tablet core of this 25mg cinacalcet tablet is made of the following components by weight percentage: 27.55% cinacalcet hydrochloride; 37.47% pregelatinized starch; 24.98% microcrystalline cellulose; 4% Polyvinylpyrrolidone; 4% cross-linked polyvinylpyrrolidone; 1% colloidal silicon dioxide; 1% magnesium stearate; see Table 1 for details.
[0025] The preparation method of this 25mg cinacalcet tablet has the following steps:
[0026] ① 27.55mg of cinacalcet hydrochloride (D 90 50 90 50 90 <250μm, intragranular), 4.00mg polyvinylpyrrolidone and 2.00mg cross-linked polyvinylpyrrolidone (intragranular) were mixed evenly.
[0027] ②Add an appropriate amount of purified water as a wetting agent, pass through a 30-mesh sieve to granulate, and put the prepared granules into an oven at 60°C to dry, and control the moisture content of the granules to ≤5%. ...
Embodiment 2)
[0032] The immediate-release formulation of cinacalcet hydrochloride in this embodiment is a 25 mg cinacalcet tablet.
[0033] The preparation method of this 25mg cinacalcet tablet is the same as that of Example 1, except that the tablet core is made of the following components by weight percentage: 27.55% cinacalcet hydrochloride; 31.20% pregelatinized starch; 26.00% microcrystalline cellulose; 4.25% polyvinylpyrrolidone; 8% cross-linked polyvinylpyrrolidone; 1.50% colloidal silicon dioxide; 1.50% magnesium stearate; see Table 1 for details.
Embodiment 3)
[0035] The immediate-release formulation of cinacalcet hydrochloride in this embodiment is a 75 mg cinacalcet tablet.
[0036] The preparation method of this 75mg cinacalcet tablet is the same as Example 1, and the difference is that the tablet core is made of the following components by weight percentage: 41.325% cinacalcet hydrochloride; 28.005% pregelatinized starch; 18.67 % microcrystalline cellulose; 4% polyvinylpyrrolidone; 6% cross-linked polyvinylpyrrolidone; 1% colloidal silicon dioxide; 1% magnesium stearate; see Table 1 for details.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com