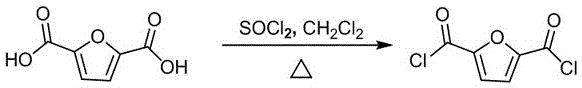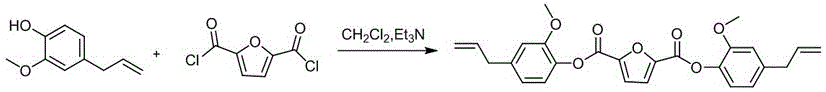Modified bismaleimide resin and preparation method thereof
A bismaleimide resin, bismaleimide technology, applied in organic chemistry and other directions, can solve the problem that the BEG conversion rate is only 60%, the thermal performance and flexural modulus are reduced, and the crosslinking density of the cured product is reduced, etc. problems, to achieve the effect of easy industrial production, excellent thermal properties and rigidity, and high crosslinking density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Preparation of 2,5-furandicarboxylic acid chloride
[0032] See attached figure 1 , it is the synthetic reaction formula of preparing 2,5 furandicarboxylic acid chloride in the present embodiment; Concrete reaction condition is as follows:
[0033] Mix 31.20g of 2,5-furandicarboxylic acid, 35.69g of thionyl chloride and N,N-dimethylformamide (DMF, catalyst, 0.05mL), stir and react at 80°C for 3h, and cool naturally After reaching room temperature, thionyl chloride was evaporated in vacuo, and after drying, 2,5-furandicarboxylic acid chloride was obtained with a yield of 99.5%.
[0034] 2) Preparation of bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass
[0035] See attached figure 2 , which is the synthesis reaction formula for preparing bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass in this example; the specific reaction conditions are as follows:
[0036] Dissolve 31.20g of eugenol and 24.29g of triethylami...
Embodiment 2
[0046] 1) Preparation of 2,5-furandicarboxylic acid chloride
[0047] Mix 31.20g of 2,5-furandicarboxylic acid, 35.69g of thionyl chloride and N,N-dimethylformamide (DMF, catalyst, 0.05mL), stir and react at 80°C for 3h, and cool naturally After reaching room temperature, thionyl chloride was evaporated in vacuo, and after drying, 2,5-furandicarboxylic acid chloride was obtained with a yield of 99.5%.
[0048] 2) Preparation of bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass
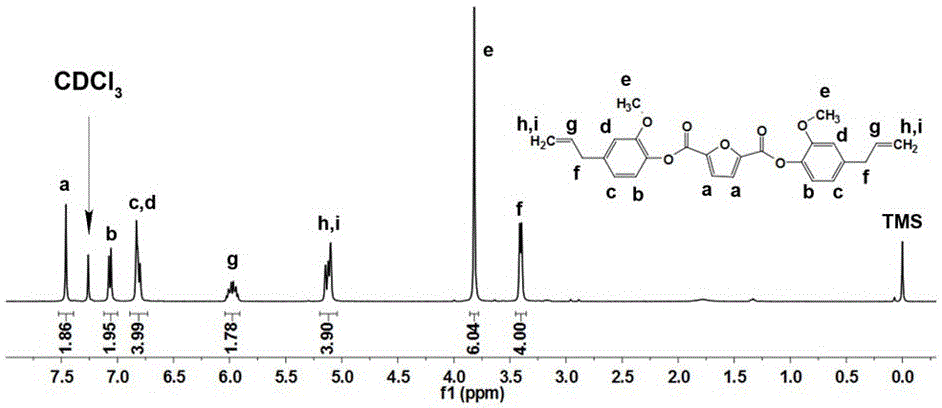
[0049] Dissolve 32.84g of eugenol and 27.33g of triethylamine as a base in 300mL of dichloromethane and stir, and drop into a solution of 2,5 furandicarboxylic acid chloride (19.30g) in dichloromethane (300mL) at -2.5±1°C After the dropwise addition, the reaction solution was slowly raised to 20°C, and the reaction was continued for 3 hours; after the reaction, the dichloromethane was evaporated in vacuo, washed with deionized water, and dried to obtain bis(4-allyl- 2-methoxy...
Embodiment 3
[0053] 1) Preparation of 2,5-furandicarboxylic acid chloride
[0054] Mix 31.20 g of 2,5 furandicarboxylic acid, 35.69 g of thionyl chloride and N,N-dimethylformamide (DMF, catalyst, 0.05 mL) at 70 o The reaction was stirred under the condition of C for 3 h, cooled naturally to room temperature, thionyl chloride was evaporated in vacuum, and after drying, 2,5 furandicarboxylic acid chloride was obtained with a yield of 99.6%.
[0055] 2) Preparation of bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass
[0056] Dissolve 34.48g eugenol and 30.36g triethylamine as base in 500mL dichloromethane and stir at -1±1 o Add a solution of 2,5-furandicarboxylic acid chloride (19.30g) in dichloromethane (500mL) dropwise at C, and after the dropwise addition, the reaction solution slowly rises to 20°C, and the reaction is continued for 4 hours; methane, washed with deionized water, and dried to obtain bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate in a yield o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| flexural modulus | aaaaa | aaaaa |
| flexural modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com