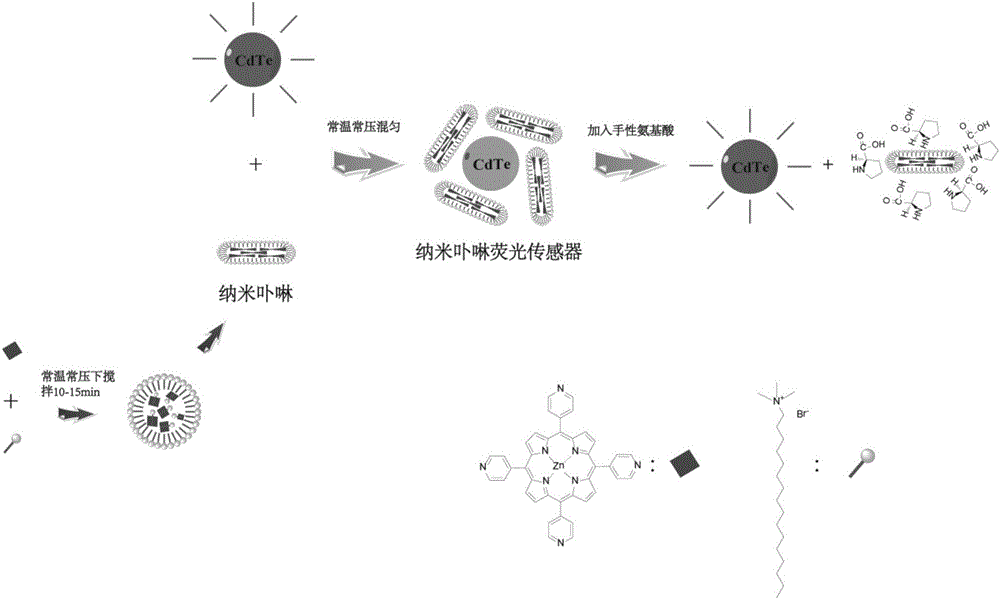
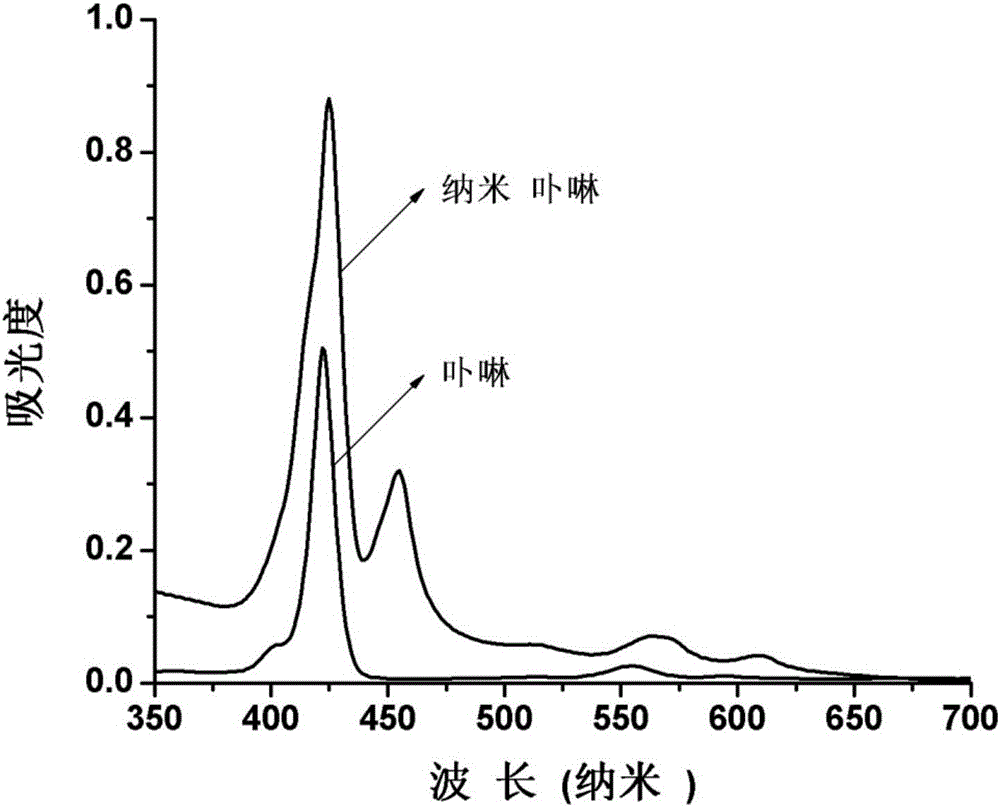
Method for recognizing quantitative chiral amino acid by using reversible nano porphyrin fluorescence sensor
A fluorescent sensor and chiral amino acid technology, applied in fluorescence/phosphorescence, material analysis through optical means, instruments, etc., can solve the problems of complex sample preparation, unavoidable derivative reagents, long detection time, etc., and achieve high sensitivity , fast response, simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: The recognition and quantitative analysis of the chirality of proline by the reversible nano-porphyrin fluorescent sensor, the schematic diagram of the method is shown in Figure 1, and the steps are as follows:
[0045] (1) Synthesis of CdTe quantum dot fluorescent probe
[0046] Dissolve cadmium dichloride (0.1142g, 12.5mM) and N-acetyl-L-cysteine (0.0979g, 15mM) in 40mL of ultrapure water, stir at room temperature and pressure for 15 minutes, and then oxidize with hydrogen The pH of the solution was adjusted to 8.00 with sodium solution, and then filled with nitrogen and stirred in an ice bath for 20 minutes. Add sodium tellurite (0.0216g, 2.5mM) and stir for 15 minutes; then add sodium borohydride (0.0113g, 7.5mM) and stir for 15 minutes. Finally, the solution was put into a reaction kettle and reacted in an oven at 200° C. for 50 minutes. Cooling to room temperature yielded 2.9×10 -7 mol / LCdTe quantum dot fluorescent probe.
[0047] (2) Synthesis of...
Embodiment 2
[0053] Example 2: The recognition and quantitative analysis of the chirality of lysine by the reversible nano-porphyrin fluorescent sensor, the schematic diagram of the method is shown in Figure 1, and the steps are as follows:
[0054] (1) Synthesis of CdTe quantum dot fluorescent probe
[0055] The CdTe quantum dot fluorescent probe was synthesized by the method of step (1) in Example 1.
[0056] (2) Synthesis of tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution
[0057] The method of step (2) in Example 1 was used to synthesize tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution.
[0058] (3) Preparation of switch nanoporphyrin fluorescent sensor
[0059] The nano-porphyrin fluorescence sensor was prepared by the method of step (3) in Example 1.
[0060] (4) Recognition and quantitative analysis of D- / L-lysine by reversible nanoporphyrin fluorescent sensor
[0061] Add 100μL- / L-lysine aqueous solution to 1.5mL cuvette, 70μL 3.42×10 -6 mol / L tetrakis-(4-pyri...
Embodiment 3
[0062] Example 3: The recognition and quantitative analysis of the chirality of serine by the reversible nano-porphyrin fluorescent sensor, the schematic diagram of the method is shown in Figure 1, and the steps are as follows:
[0063] (1) Synthesis of CdTe quantum dot fluorescent probe
[0064] The CdTe quantum dot fluorescent probe was synthesized by the method of step (1) in Example 1.
[0065] (2) Synthesis of tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution
[0066] The method of step (2) in Example 1 was used to synthesize tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution.
[0067] (3) Preparation of switch nanoporphyrin fluorescent sensor
[0068] The nano-porphyrin fluorescence sensor was prepared by the method of step (3) in Example 1.
[0069] (4) Recognition and quantitative analysis of D- / L-serine by reversible nanoporphyrin fluorescent sensor
[0070] Add 100μL- / L-serine aqueous solution to 1.5mL cuvette, 70μL 3.42×10 -6 mol / L tetrakis-(4-py...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com