Application of melatonin to preparation of medicine for treating loose periprosthetic chronic inflammation and bone destruction
A technology for periprosthesis and chronic inflammation is applied in the field of melatonin to prepare medicines for treating chronic inflammation and bone destruction around loose prostheses, and achieves the effects of preventing and treating prosthesis loosening, reducing bone destruction and having definite curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Main drugs and reagents
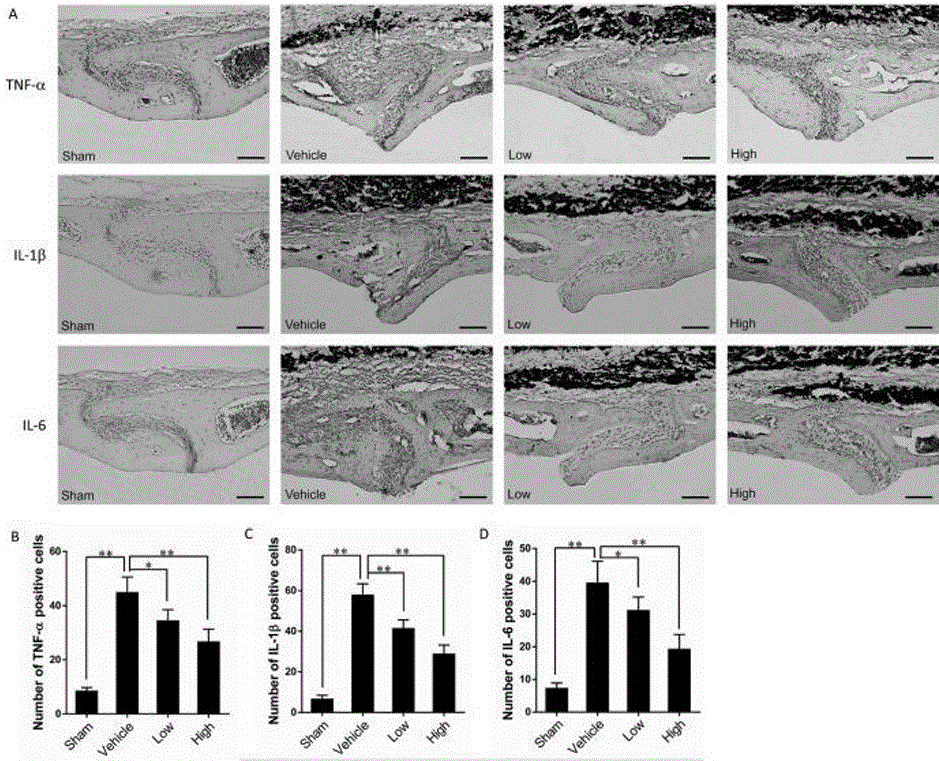
[0023] Melatonin (Mel), TRAP staining kit, purchased from Sigma, USA; paraformaldehyde, PBS, DAB chromogen, hematoxylin, eosin, absolute ethanol, distilled water, 10% chloral hydrate. Titanium particles were purchased from Johnson Mattheychemicals (catalog #00681; Ward Hill, Massachusetts); RANKL, OPG, TNF-α, IL-1β, IL-6 ELISA detection kits were purchased from Biosource, USA; OPG , RANKL, β-catenin, DKK1, TNF-α, IL-1β, IL-6 antibodies were purchased from Abcam, UK.
[0024] 2. Main instruments
[0025] Micro-CT (SkyScan 1176, Belgium), paraffin slicer (Leica 2135, Germany), slicer (Leica 1120, Germany), paraffin embedding machine (BMJ-Ⅱ, Changzhou, China), Axiovert 40C optical microscope (Zeiss, Germany), a microplate reader (Biotec, USA), and a set of surgical instruments.
[0026] 3. Experimental animals
[0027] Sixty healthy C57BL / J6 mice, male, weighing 19-22 g, 8-10 weeks old, clean grade, were provided by the Animal Experiment Ce...
Embodiment 2
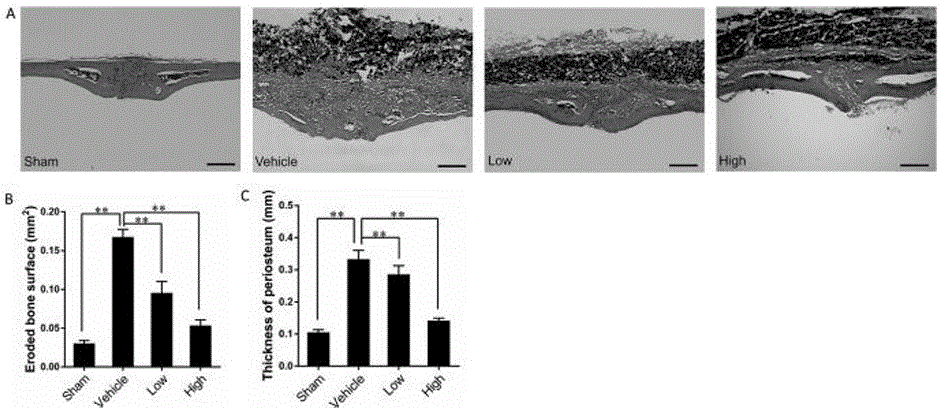
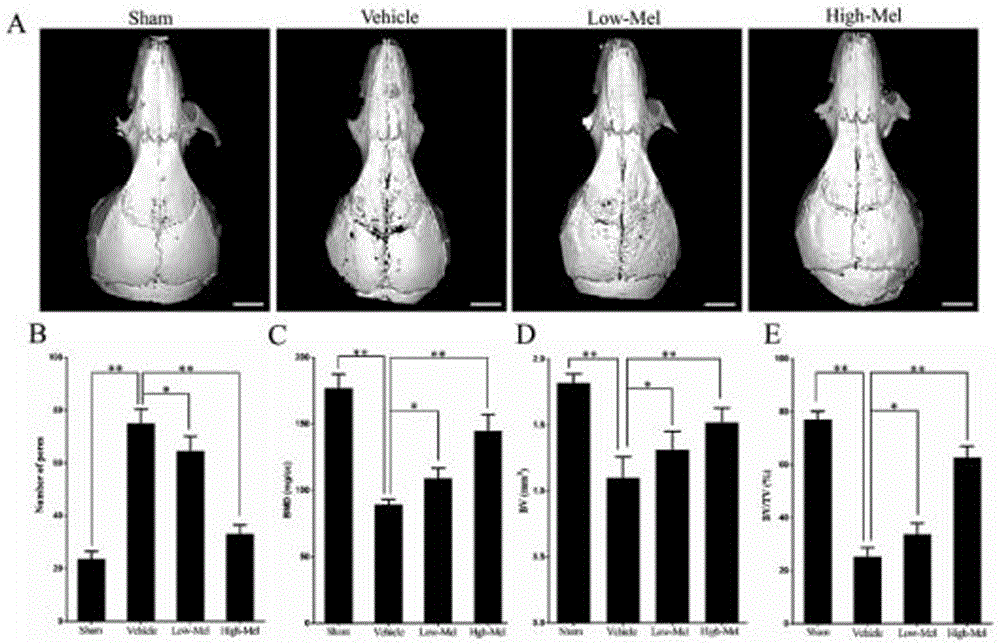
[0038] The high-resolution micro-CT SkyScan 1076 produced by Belgium SkyScan Company was used to scan and analyze the mouse skull. Remove the specimen from the fixative and allow to dry before scanning. Referring to the method of Zhai et al., the black Ti particles on the surface of the skull were gently removed with a scalpel blade to avoid the interference of metal artifacts during scanning. Place each skull in a micro-CT test tube cup, 5 at a time, and separate each skull with a foam sheet; the skulls should be placed neatly to avoid touching the wall of the test tube. The scanning parameters were set as follows: scanning resolution 18 μm, rotation angle 180°, rotation angle increment 0.9°, voltage 80 kV, current 100 μA, exposure time 100 ms. After scanning, the SkyScan 1076 built-in software was used for 3D reconstruction of the skull. According to the method established by Wedemeyer, use CT Analyzer analysis software (CT An, SkyScan) to select a cylindrical region of in...
Embodiment 3
[0040] 1. Bone Tissue Staining and Morphometric Analysis
[0041] Handling of tissue samples
[0042] (1) Specimen fixation: wash the fresh skull specimens and soak them in 10% paraformaldehyde solution for 48 hours;
[0043] (2) Decalcification of the specimen: Put the fixed skull into a container filled with 10% EDTA decalcification solution (pH 7.4), decalcify at room temperature for 3 weeks, and change the solution every 3 days. The method of judging the success of decalcification: the needle of the syringe can pass through the skull without resistance, that is, the decalcification is successful;
[0044] (3) Specimen washing: After successful decalcification, rinse the specimen under running water for 2 hours to completely remove the components of the decalcification solution;
[0045] (4) Specimen dehydration: Pass the rinsed specimen through 50% ethanol, 60% ethanol, 70% ethanol, 80% ethanol, 90% ethanol, 95% ethanol I, 95% ethanol II, absolute ethanol I at room tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com